Craniofacial Developmental Biology Lab
Contact Information
Craniofacial Developmental Biology Laboratory
Eric C. Liao, MD, PhD, Principal Investigator
Simches Research Building
185 Cambridge Street
Boston,
MA
02114
Phone: 617-643-5975
Email: cliao@partners.org
Accessible
Near Public Transit
Explore This Laboratory
Overview
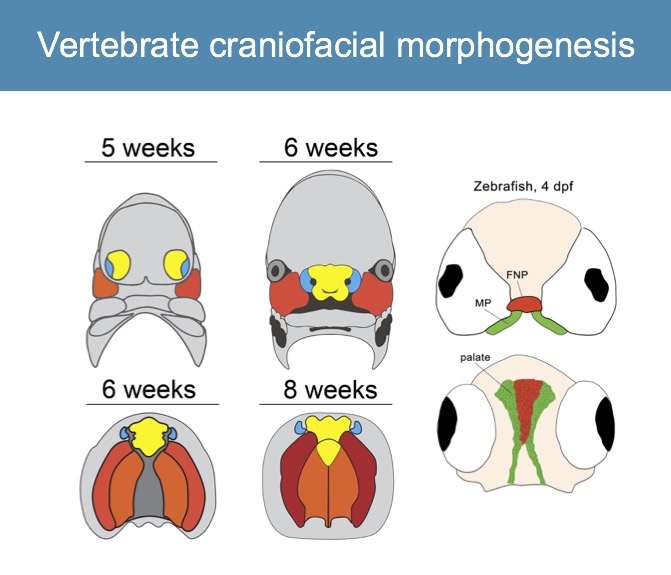
Formation of the vertebrate facial structures requires coordination of complex molecular and morphogenetic cues. The genes regulating facial development are well conserved across vertebrate species, where minor molecular variations contribute to dramatic alterations in form.
We take advantage of the versatility of forward and reverse genetics in zebrafish as a model to assay function of human cleft candidate genes and demonstrated that the zebrafish palate (ethmoid plate) is morphogenetically homologous to the mammalian primary palate (Dougherty, Development, 2013). We also showed that convergence and extension mechanisms operate in palate morphogenesis, and we are dissecting the Wnt pathway genetically (Rochard, Development, 2016; Kamel, Developmental Biology, 2013).
Advances in clinical treatment of congenital craniofacial malformations require improved understanding of the developmental genetic basis of facial morphogenesis. Our goal is to investigate fundamental genetic regulation of facial development, with focus on translating basic science discoveries to clinical treatments.
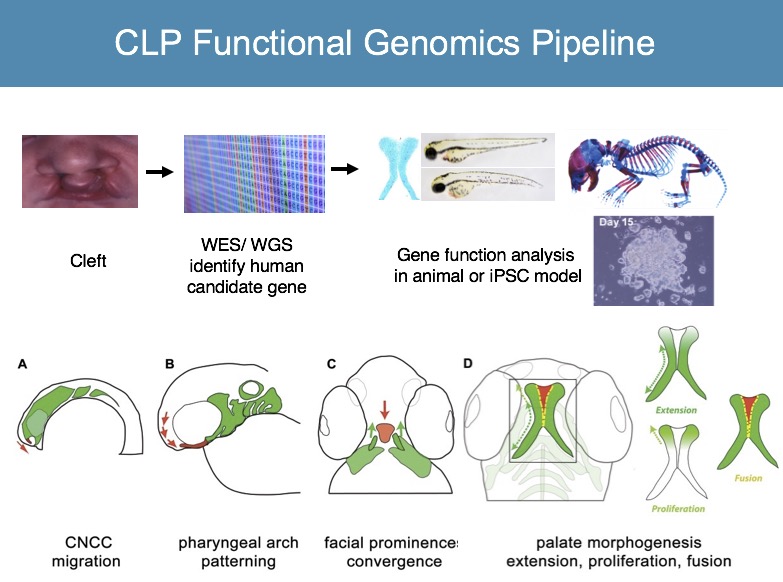
We are applying zebrafish to carry out high throughput functional genomics studies, to characterize human genes implicated in orofacial clefts (Mukherjee, Human Molecular Genetics, 2016; Gfrerer, Plastic and Reconstructive Surgery, 2014). We are also using zebrafish as the biological platform to identify chemicals that specifically regulate craniofacial morphogenesis, or even discover compounds that mitigate malformations (Kong, Chemistry & Biology, 2014).
With continued revolutionary advance in human gene sequencing approaches, there is pressing need to bridge the gap between whole genome analysis and clinical use of this data. Determination of pathogenicity of human gene variants is a major challenge in the field, limiting clinical usefulness of WGS information. We apply functional assays in iPSC and zebrafish models toward functional analysis of human gene variants associated with cleft and craniofacial anomalies (E BH Li, Plos Genetics, 2017).
Research Projects
Fundamental and Translational Research
Our research projects include:
- Cranial neural crest biology
- High throughput functional analysis of candidate genes implicated in facial morphogenesis
- Neural crest biology of craniofacial development
- Tissue engineering and vascularized constructs
- Zebrafish models of cleft lip and palate malformation
- Wnt signaling in craniofacial development
Clinical Research
- Breast reconstruction outcomes
- Cleft care, fetal diagnosis and congenital personal medicine
Research Positions
Post-doctoral research fellowship positions are available; please arrange with Eric Liao, MD, PhD. Funding can be guaranteed for the candidate with appropriate experiences and references.
Job Description
The Liao Laboratory at the Center for Regenerative Medicine at Massachusetts General Hospital in affiliation with the Division of Plastic and Reconstructive Surgery and the Harvard Stem Cell Institute is seeking a highly motivated research fellow interested in craniofacial developmental biology and malformations. The laboratory has innovated application of the zebrafish model toward the study of human craniofacial anomalies, contributing recent papers on zebrafish cleft models and cranial neural crest lineage maps. The fellow will join a collaborative group of scientists who study the basic and translational biology of facial morphogenesis, cranial neural crest specification and development. In this instance, the fellow’s work will have a direct bearing on discovery of drugs to mitigate malformation phenotypes and functional genomics of human candidate genes implicated in orofacial clefts. We are also inherently interested in the basic biology of neural crest cell specification, migration, differentiation and potential for regeneration. We integrate the approaches of classical developmental and modern stem cell biology and use these to explore human tissue regeneration and disease. We primarily work with the zebrafish model with collaborators in human and mouse genetics. We apply the latest technology in zebrafish genetics with routine use of TALENs and CRISPR, morpholinos, cell transplants, FACS, live confocal labeling and imaging, transgenics, chemical screening, and many other experimental approaches are routine. Our laboratory has a dedicated zebrafish facility outfitted with the latest imaging, injection and cell manipulation instruments. Researchers work in open format laboratory space that foster interactions with other stellar researchers working in CRM, with ample opportunities to participate in scientific seminars and professional development.
Job Requirements
The candidate should have PhD or an MD/PhD in a relevant field or equivalent training. A strong foundation in cell, developmental, or cancer biology with strong skills in molecular biology is necessary, including a foundation in the genetic modulation of a model organism. Prior experience working in zebrafish genetics is strongly desired but not a requirement. An ancillary knowledge of genomics or systems biology is welcome. Knowledge of human and clinical genetics is unnecessary, as it will be acquired rapidly within the laboratory environment. Outstanding oral and written communication skills are required.
Please email Eric Liao your:
- CV
- Statement of interest and career goals
- Names and contact information of at least three (3) references
Publications
Purification, culture and multi-lineage differentiation of zebrafish neural crest cells
Experimental Biology and Medicine, February 2014
Group Members
Meet the team that makes up the Craniofacial Developmental Biology Laboratory.
Video Gallery
Cranial neural crest cell migration in the Tg:sox10::kaede transgenic, wildtype, 16 somite to 24 hpf
Cranial neural crest cell migration in the Tg:sox10::kaede transgenic, wildtype, approx 2.5-3.5 dpf
Cranial neural crest cell migration in the Tg:sox10::kaede transgenic, wildtype, approx 48-54 hpf
Contact Us
Our goal in the Craniofacial Developmental Biology Laboratory is to investigate fundamental genetic regulation of facial development, with a focus on translating basic science discoveries to clinical advances.
