MUCOSAL IMMUNOLOGY & BIOLOGY RESEARCH
Göbel Lab: Verena Göbel, MD
Contact Information
Laboratory of Verena Göbel, MD
Building 114, 16th Street
Charlestown,
MA
02129
Phone: 617-643-4491
Email: vgobel@mgh.harvard.edu
Physician Investigator, Pediatric Hematology and Oncology, Massachusetts General Hospital
Associate Professor of Pediatrics, Harvard Medical School
Pediatrician, Pediatrics, Massachusetts General Hospital
Explore This Lab
Overview
One of the objectives of the Laboratory of Verena Göbel, MD, at Massachusetts General Hospital is to contribute to the understanding of the molecular basis of intestinal morphogenesis. C. elegans is a transparent roundworm whose internal organs are formed by different types of tubes constructed from distinct, yet simple, polarized epithelia.
The simplicity of this organism, when combined with its sophisticated genetic resources, makes it a powerful tool to examine tubulogenesis.
We have recently identified a number of vesicle-associated proteins and lipids that are required for apicobasal plasma membrane domain and lumen foundation.
Understanding organ development will advance our understanding of the pathogenesis of human diseases related to these organs, which in turn should lead to novel approaches for their diagnosis and treatment. Specifically, we hope that this work will translate into a better understanding of:
- The still-enigmatic link between the development of cancer and the disruption of polarity, cell shape and developmental genes
- Developmental diseases of internal organs and the vasculature
Research Projects
Polarity and Tubulogenesis
The breaking of symmetry—or the establishment of polarity—is one of the ancient and still unresolved mysteries of biology. Polarity is a prerequisite for spatial diversity, growth, development and homeostasis of all multicellular organisms.
Having identified thousands of genes and molecular interactions in recent years, we now have the ability to ask which molecules generate and maintain an asymmetric state and how they accomplish this.
We investigate how polarity is regulated during development, and how it determines the formation of complex three-dimensional structures (particularly tubes) during growth. Tubes are building blocks of all internal organs, whose shape and function depend on their intact polarity.
They are composed of polarized epithelial cells with their apical domains generating the lumen (the functional interface with the external environment), and their basolateral domains contacting adjacent cells and the extracellular matrix.
C. elegans is a small transparent roundworm with simple single-layered epithelia that allows the microscopic observation of polarized membrane biogenesis at single-cell resolution in a three-dimensional in vivosetting during organ morphogenesis (Figure).
C. elegans is also a classical genetic model organism with a track record of discovering novel basic biological mechanisms that also operate in humans. In C. elegans, morphogenesis can be observed during embryonic development and animals can be engineered such that distinct organ surfaces fluoresce in different colors—all things that cannot be done in humans. Importantly, genetic screens--one of the few ways to determine how biological processes work on a molecular level—can be performed without any preexisting bias as to what the answer will be.
Our C. elegans screens therefore have the potential to identify, among the many thousands of genes, those that are indispensable to the process being examined (here, organ morphogenesis).
We have conducted genome-wide RNA interference screens on multicellular (intestinal) and unicellular (excretory system) tubulogenesis by knocking down single genes and examining the consequences of the loss of each on polarity and organ formation in worms with fluorescing tubes and lumenal membranes. These screens have identified novel polarity and “tube” phenotypes, some of which remarkably resemble those seen in human diseases.
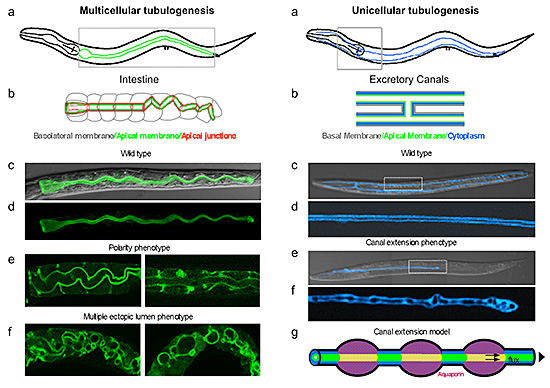
Unicellular Tubulogenesis: (a, b) The C. elegans excretory canals, running along the length of the animal, are extended from one single cell. Boxed area in (a) is magnified in (b). The apical membrane (green) defines the lumen. (c, d Wild-type adults with fluorescent canals (pseudo colored to blue), fully extended. Lumen (color-excluded stripe) is contiguous (magnified in d). (e, f) Mutant canal extended to ½ body-length, lumen is non-contiguous (magnified in f). Confocal/Nomarski overlays in c, e, confocal projections in d, f. (g) Aquaporin-regulated rhythmic fluid shifts propel directional lumen and canal extension.
For instance, an intriguing intestinal polarity and multiple-ectopic-lumen phenotype (Figure) closely mimics the intestinal defects observed in Microvillus Inclusion Disease (MVID), an inherited neonatal intestinal failure syndrome with multiple intracytoplasmic lumens in the intestine.
The characterization of the underlying gene products have identified unexpected roles in tubular polarity of well-known molecules that were previously recognized for either related or distinct functions in mammalian cell lines.
An intestinal tubulogenesis screen, for example, identified glycosphingolipids (proposed lipid raft components) as apicobasal membrane domain determinants in multicellular tubulogenesis;1 and it discovered an unexpected role for the classical vesicle-coat clathrin and its AP-1 adaptor in apical polarity and intestinal lumen morphogenesis.2
Loss of these genes generates an MVID-like phenotype in worms, demonstrating the compatibility of the invertebrate and vertebrate systems and identifying these genes as novel candidate MVID (or related intestinal development failure) genes.
An excretory canal tubulogenesis screens, on the other hand, identified a water channel (aquaporin) as being required to propel the extension of a unicellular tube by translumenal flux, a novel morphogenetic process that had not yet been demonstrated to directly shape tissues during animal development3 (Figure).
This study also revealed that the membrane-cytoskeleton linker ERM-1, the ortholog of a mammalian regulator of cortical membrane dynamics, is strictly required for the de novo expansion of a lumenal membrane.
These findings have implications for the development of human capillary-type tubes that generate part of the vascular system.
We are currently investigating additional intriguing genes that were also identified in these screens. We hope to assemble them into a coherent pathway that will molecularly characterize a novel mechanism for the establishment and maintenance of membrane polarity and reveal how these genes are coordinated during the process of tube, lumen and organ morphogenesis.
The equivalent human genes of some of these molecules have already been implicated in tumor development, suggesting that this analysis will also identify novel cancer-related genes and their respective roles in polarity and morphogenesis.
Citations
1. Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Göbel V. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Bio 2011; 13(10): 1189 – 1201
2. Zhang H, Kim A, Abraham N, Khan LA, Hall DH, Fleming JT, Göbel V. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development 2012; 139(11): 2071-2083.
3. Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, Hall DHH, Göbel V. ERM-1/ezrin- radixin-moesin interacts with AQP-8/Aquaporin in intracellular lumen and unicellular tube extension. Nat Cell Bio 2013; 15(2):143-156.
Publications
Selected Publications
- Göbel V, Barrett PL, Hall DH, Fleming JT. Lumen morphogenesis in elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell 2004; 6: 865-873.
- Barrett PL, Fleming JT, Göbel V. Targeted gene alteration in Caenorhabditis elegansby gene conversion. Nat Genetics 2004; 36: 1231-1237.
- Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Göbel V. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Bio 2011; 13(10): 1189 - 1201.
- Zhang H, Kim A, Abraham N, Khan LA, Hall DH, Fleming JT,Göbel V. Clathrin and AP-1 regulate apical polarity and lumen formation during elegans during tubulogenesis. Development 2012; 139(11): 2071-2083.
- Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, Hall DHH, Göbel V. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat Cell Bio 2013; 15(2): 143-156.
- Zhang H, Kim A, Abraham N, Khan LA, Göbel V. Vesicular sorting controls the polarity of expanding membranes in the Elegansintestine. The Worm 2013.
