Chiari I Cerebellar Function Study
Request an Appointment
Contact our nurse practitioner, Elizabeth Shannon, to learn more
Research Consent Form (English / Spanish)
Research Assent Form (English / Spanish)
There is an open human study at Massachusetts General Hospital whose purpose is to develop a better understanding of the broad range of neurological symptoms that a person with Chiari type I malformation can experience. If you are a symptomatic Chiari I patient who is scheduled for decompressive posterior fossa (commonly referred to as the post fossa) surgery by William E. Butler, MD, neurosurgeon in the Department of Neurosurgery, then you may be a candidate for this study. This study has been approved for enrollment starting July 2020 by the Mass General ethics committee for research involving human subjects. This study is motivated by an inability to explain many of the presenting symptoms suffered by Chiari I patients.
If you choose to be a part of this study, you may also be a candidate for a neuroimaging study whose purpose is to better understand how imaging tools can be used to study cerebellar function. This additional neuroimaging study entails an MRI and magnetoencephalography (MEG) scan on a separate visit to the Athinoula A. Martinos Center for Biomedical Imaging. Because this would require another visit, you will be given a reimbursement of $100 for participating in the neuroimaging study.
The conventional understanding of the cerebellum is that it is involved in balance and coordination. However, many persons with symptomatic and intractable Chiari I malformation have cognitive and affective impairments beyond physical balance and coordination. For example, they may describe reduced clarity of thought, reduced memory, reduced capacity for sensory integration and many other cognitive and affective phenomena that are hard to characterize. They commonly use the phrase “brain fog“ to describe their mental and emotional state. These phenomena lead one to ask if the cerebellum may play a role in cognitive, speech and emotional processing, and whether these symptoms may reflect alterations in cerebellar processing in patients with symptomatic Chiari I malformation.
As a morphological disease condition, Chiari I is rooted in a disproportionality of the volume of the post fossa compared to the contained brain structure. These brain structures include the brainstem and the cerebellum. The cerebellum is a brain structure within the lower back part of the head as illustrated on the left side of Fig. 1. This compartment in the lower back part of the head is called the post fossa. There is a hole through the bone in the bottom of the post fossa called the foramen magnum. This is the passageway through which the brainstem exits and becomes the spinal cord within the spinal canal. In the standard anatomical configuration as on the left side of Fig. 1, the cerebellum and the brainstem are bathed in spinal fluid in the vicinity of the foramen magnum.
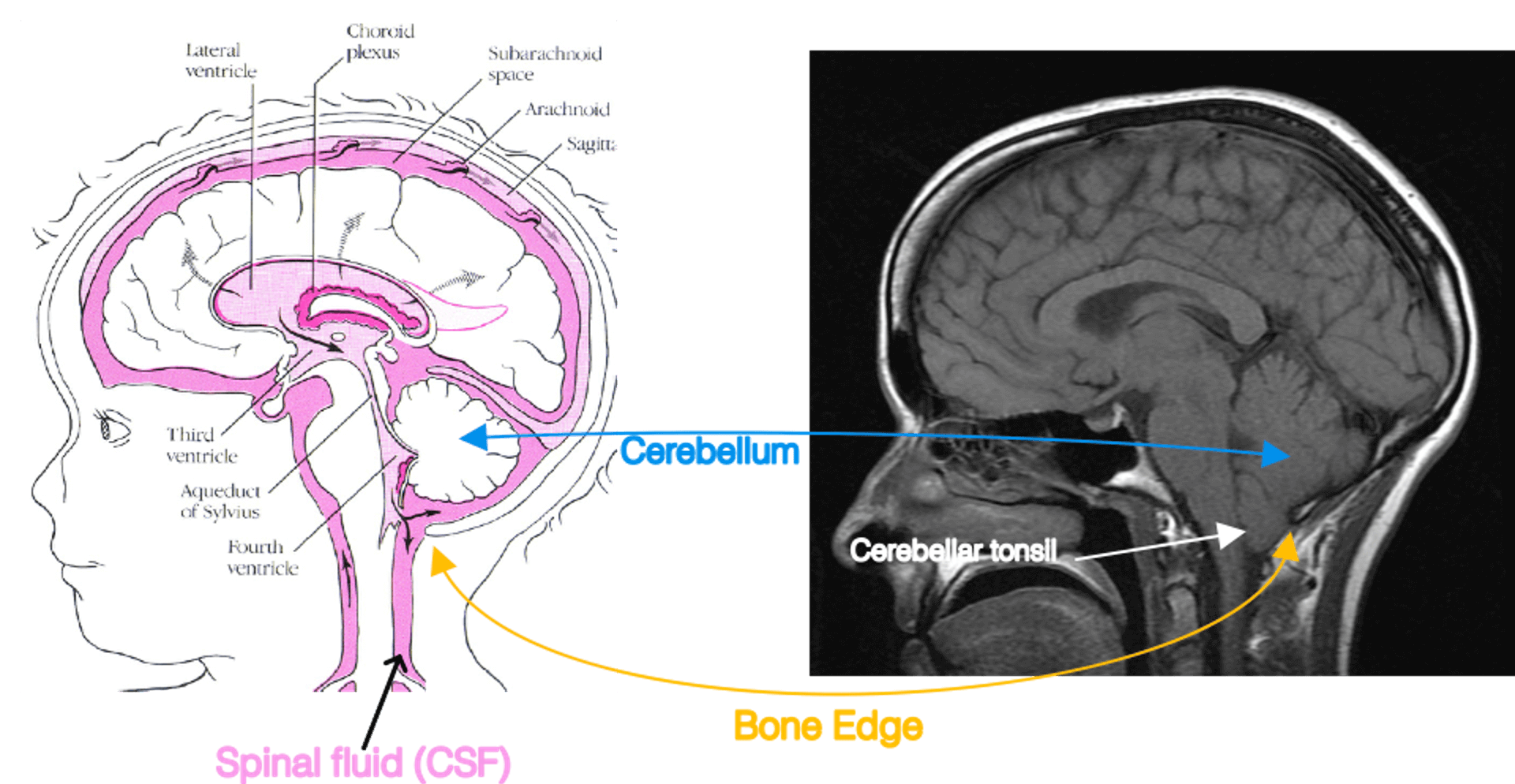 Fig. 1. The cerebellum and its compression in Chiari I. Left: A diagram adapted from the Hydrocephalus Association. Right: A teaching file mid-sagittal MRI of Chiari I. In Chiari I, the cerebellum is compressed by abnormal bone formation. This causes the cerebellar tonsil to extrude into the spinal canal.
Fig. 1. The cerebellum and its compression in Chiari I. Left: A diagram adapted from the Hydrocephalus Association. Right: A teaching file mid-sagittal MRI of Chiari I. In Chiari I, the cerebellum is compressed by abnormal bone formation. This causes the cerebellar tonsil to extrude into the spinal canal.In Chiari I malformation, the post fossa compartment is comparatively too small for the brain tissue volume occupied by the cerebellum and brainstem. Some of these findings are shown on the right side of Fig. 1, which is a midline sagittal T1 image of a symptomatic Chiari patient adapted from a human brain MRI teaching file. As a consequence of the comparatively small post fossa case volume compared to the brainstem and cerebellum, the bottom part of the cerebellum (called the cerebellar tonsils) and the brainstem are crowded in the foramen magnum. There is reduced or even absent spinal fluid bathing them at the foramen magnum. The cerebellar tonsils may exit out of the skull cavity and into the top posterior part of the spinal canal.
There is much that remains unknown about the functions of the cerebellum. Although it is only about 11% of the human brain by weight, it holds about 70% of the brain's neurons. It appears to play a critical role in many brain processes, including coordination of motion and balance, processing of sensory input from the body, cognitive function, speech and the balance of emotion. Yet the precise role the cerebellum plays in these functions remains poorly understood. A major part of this lack of knowledge is due to a poor understanding of what other brain parts it connects to. In this study, we ask if measurement of the cerebellum’s surface electrical properties might lead to a better understanding of the role of the cerebellum in Chiari I symptoms.
There has been some progress made in imaging of the cerebellum and in cerebellar magnetic encephalopathy (MEG). As part of the effort to learn how the cerebellum participates in judgment and emotion processing, Jeremy Schmahmann, MD, director of the Ataxia Unit at Mass General, has proposed a cerebellar cognitive-affective scale.
Despite these efforts, basic facts about the cerebellum such as the nature of its connections to other areas of the brain structure remains poorly described. This study has two objectives: 1) to use electrophysiology to shed some initial light on the connectivity of the cerebellum with other parts of the nervous system and 2) to develop insight into some of the complex and poorly understood neurocognitive symptoms suffered by people with a symptomatic version of Chiari I malformation.
To be sure, in a large fraction of persons with radiological Chiari I malformation, the condition is incidental and asymptomatic. In such instances it can be managed conservatively. Some persons with Chiari I malformation present with the Chiari I symptoms. An important and relatively common Chiari I symptom is exertional headache. If the Chiari I symptoms (a) are of sufficient intensity and severity as to degrade the quality of daily life; (b) have not responded to conservative or medical therapy and (c) are slowly progressive, then such a person may be a candidate for treatment of the Chiari symptoms by post fossa decompressive surgery.
In this procedure, a person when under general anesthesia is presented facedown and the cerebellum and foramen magnum are operatively exposed and decompressed. The final step in the decompression is the incision of the dura. At surgery, this step exposes the surface of the cerebellum. It allows the cerebellum to begin to move with the heartbeat. Fig. 2 shows an approximate operative view of the surface of the cerebellum after the dural incision is made. After this final step in the decompression, the wound is closed.
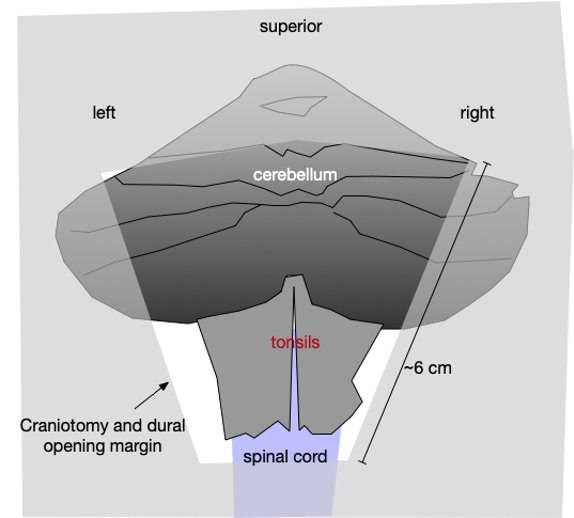
Fig 2. Cerebellar exposure upon Chiari decompression. The dural incision yields approximately this cerebellar surface exposure
To promote the safety of the procedure, while under general anesthesia, the sensory and motor neurological systems of a person undergoing cerebellar decompression for Chiari type I malformation are electrophysiologically monitored.
This neuromonitoring has two chief components. These are somatosensory evoked potentials (SSEPs) and transcranial motor evoked potentials (TCMEP).
Monitoring of the sensory system takes place via somatosensory evoked potentials (SSEP) triggered by electrical stimulation of the peripheral nerves. As such, low-level electrical stimulation is applied to the skin overlying the posterior tibial nerve (for the legs) and/or the median nerve (for the arms) and the triggered electrical volleys travel through sensory pathways towards the brain where they finally activate the sensory cortex. The resulting brain waves (i.e. SSEP) are recorded via electrodes placed on the scalp. Changes in the continuously monitored SSEP allow real-time detection of early, reversible (if acted upon) disturbance in the transmission of the sensory information across the area where the decompressive surgery is being performed. This is illustrated in Fig. 3.
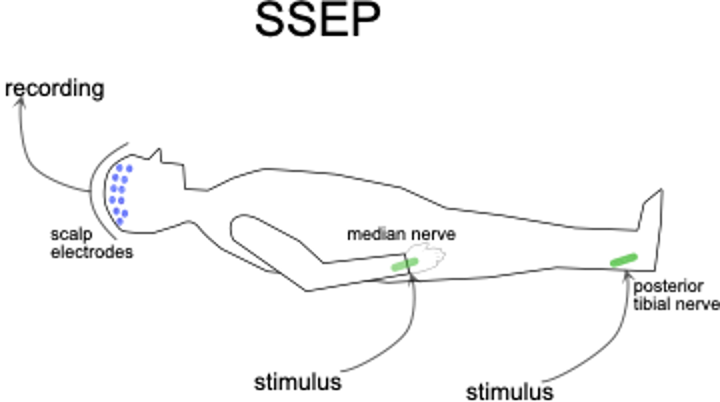
Fig. 3. Somatosensory evoked potentials. Electrical stimuli are applied to the skin over the posterior tibial and/or median nerves while scalp electrodes measure electrical potentials arising from the sensory region of the brain surface form the brain surface. If they coincide in timing, then the sensory neural circuits between the peripheral nerves and the brain surface remain in physiological continuity.
Monitoring of the motor system takes place via muscle motor evoked potentials evoked via transcranial electrical stimulation (tcmMEP). As such, electrical stimulation is applied onto the brain surface, over the motor regions, by means of electrodes placed on the scalp. This stimulation triggers electrical volleys that travel through the motor system and finally activate muscles in the face, trunk, arms and legs. The triggered electrophysiologic responses (i.e., tcmMEP) can be recorded via muscle electrodes. During the surgery, we continuously monitor such tcmMEP in real time. For as long as the latter are stable, we know that the electrical impulses from the brain to the body are being transmitted undisturbed across the area where the decompressive surgery is being performed. This is illustrated in Fig. 4.
In this study, we seek to characterize the connectivity of the cerebellum to those areas of the brain that are under monitoring via the scalp and body electrodes that correspond to the neuromonitoring. We perform the same neuromonitoring, both SSEPs and tcmMEP, as per our routine for decompressive Chiari I surgery. The addition to routine Chiari decompressive surgery is that from the exposure of the surface of the cerebellum we record electrophysiological data how the cerebellum interacts with the nervous system functions that are monitored by SSEPs and tcmMEP.
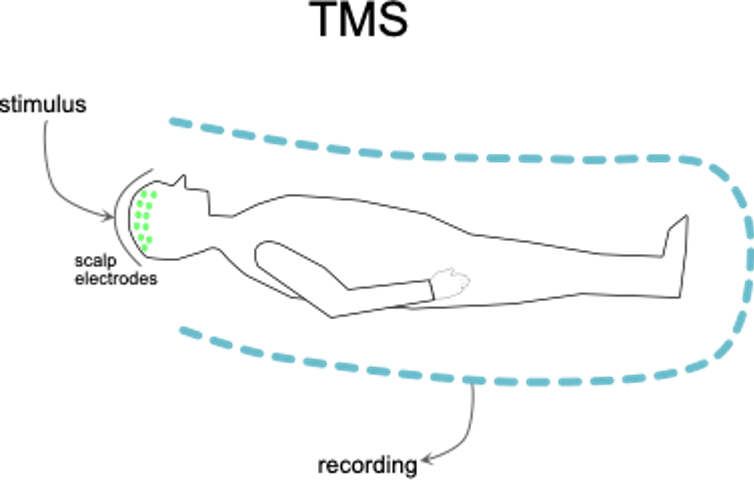
Fig. 4. Transcranial motor stimulation. Electrical stimuli are applied to the brain surface via electrodes on the scalp. If this produces motions in the head, trunk and limbs that coincide in timing, then the motor neural circuits between them remain in physiological continuity.
We record the extra data by means of sterile soft electrodes that are placed on the surface of the cerebellum. These are the same types of electrodes that have been used to study brain surface function in other areas of neurosurgery. For example, in persons with brain tumors near speech areas, the exposure of the tumor vicinity is performed under local anesthesia with the person remaining awake so that speech may be evaluated throughout the procedure. Electrical recordings of brain surface function are made by these electrodes and in addition, the influence on speech by the application of electrical stimulation to the exposed brain surface is evaluated. The experience with brain surface electrical studies over the course of decades has resulted in techniques for using these soft electrodes that have a high track record of safety and reliability.
The data acquisition protocol in the study then is as follows:
The decompressive Chiari I surgery starts and proceeds as per routine up to and including the final decompressive step, which is the opening of the dura over the cerebellum and foramen magnum. Before the decompressive incision, the dura here is commonly tense and does not have normal to-and-fro motions with the heartbeat. Upon the dural incision and decompression of the cerebellum, the cerebellum and adjoining structures then commence to move with the heartbeat. This cardiac frequency motion of the cerebellum and its surrounding cerebrospinal fluid (CSF) is an important signal to the surgeon that the decompressive portion of the procedure is complete and that closure of the wound may commence. This is the stage where the electrical connectivity of the cerebellum is studied for a maximum of 15 minutes. The initial step in closure of the wound is the suturing of a dural graft. It is placed to cover the exposed cerebellum from the opening of the tense dura.
To protect privacy, the electrophysiological data labeled with a synthetic identifier and stored in a secure location behind the Mass General firewall. The analysis of the data does not occur during the surgery. The conclusions, if any, will await the acquisition of data in similar circumstances from a sequence of persons undergoing carry surgery and will then be published in the peer-reviewed scientific literature. This data will not be of clinical value to an individual person who participates.
This study has been approved by the Mass General ethics committee for research involving human studies. The committee regarded the risks of this as small in relation to the potential contribution to general knowledge of the functions of the cerebellum. Those risks include whatever consequences there may be from extending the duration of the surgery for up to 10-15 minutes and the risk, albeit small, from the application of sterile electrodes to the cerebellar surface for the electrophysiology studies.
Neuroimaging Study
Patients that choose to participate in the cerebellar function study also has the option to participate in the neuroimaging study. Participation in the neuroimaging study is completely optional and patients can choose to be a part of the cerebellar function study but not the neuroimaging study. The purpose of this study is to understand the extent to which cerebellar function can be measured with electro- and magnetoencephalography (M/EEG).
M/EEG are electrophysiological measurement techniques where magnetic fields (MEG) and electric potentials (EEG) are measured around the head. M/EEG are completely non-invasive entailing no exposure to radiation or injections of any sort.
In this study, study participants will come to the the Athinoula A. Martinos Center for Biomedical Imaging at the Charlestown Navy Yard campus on a day prior to surgery. At the site, study participants will be scanned with an M/EEG system and then with MRI. Before the M/EEG scan, stimulating electrodes will be placed over the participant’s hand and foot in addition to the recording electrodes placed on the scalp. The participant will then be asked to sit in the MEG scanner while the stimulating electrodes on the hand and foot give a weak electrical stimulation that should not be painful or uncomfortable but sufficiently strong to cause a small twitch. These stimuli are similar to the ones used to elicit somatosensory evoked potentials described above. Meanwhile, the M/EEG system will be measuring the participant’s brain responses to these stimuli. These recorded data will be saved and securely stored at the Martinos center computer system. These data will then be compared with the electrophysiology data from the cerebellar cortex recorded during surgery. By this comparison we hope to be able to answer whether cerebellar electrophysiology can be measured with M/EEG.
There is no benefit to a person undergoing Chiari I decompressive surgery from participating in the study. There is no payment for participating. There are no extra hospital visits. If a person is offered the opportunity to participate but wishes not to, there is no change in the care that person receives. Decompressive Chiari I surgery candidates who wish to learn more about this study are given an opportunity to discuss it with a study staff member who is not the operating surgeon.
The neuroimaging study does require an extra hospital visit which will take 2-3 hours and the study participant will receive a remuneration of $100 for this extra visit.
On this webpage, we include links to the consent forms (the neuroimaging study has separate consent forms). On this page, we include links to the consent forms. They are offered in the most common languages employed by persons under the care of Mass General for Chiari I malformation (English and Spanish); however, interpreters are available to review these materials with persons who are fluent in other languages. There are consent processes for three different age groups. The consent for participation in the study is provided to the same person who provides written consent for the clinical Chiari surgery. There are three consent age categories.
Adult Chiari patients decide and sign written consent for themselves. An adult parent or legal guardian decides and signs consent for children under the age of 12. For youth ages 12–18, there is a youth assent form. This does not have the legal meaning of the consent form that would be signed by the parent or legal guardian, but it is recognition that persons in that age group merit significant autonomy in this decision and are in a matter of years to be fully responsible for these decisions.
The Chiari-cerebellar connectivity investigators have deep experience in the relevant fields. Dr. Butler is a neurosurgeon with subspecialty focus on developmental abnormalities of the nervous system, including Chiari I malformation. His practice includes all ages, spanning from premature infants to latter adulthood. He has performed in excess of 400 Chiari decompressive surgeries. Neurosurgeon Ziv Williams, MD, PhD, has a subspecialty focus on functional neurosurgery. This area of neurosurgery focuses on the study of the electrophysiology of nerve tissue and activities between brain components. Dr. Williams is the head of the Cognitive Neuroscience Lab at Mass General. He has deep experience in functional neurosurgery research and is a recipient of the United States Presidential Scholar Award. Mirela Simon, MD, MSc, and clinical neurophysiologist who focuses on neuro-monitoring techniques. Dr. Simon has extensive experience in intraoperative neurophysiological mapping and direct brain recording. Her textbook on neuromonitoring, Intraoperative Neurophysiology: A Comprehensive Guide to Monitoring and Mapping, is a standard reference in the field. Matti Hämäläinen, PhD, who leads the neuroimaging study, is a world leader in the field and is the director of the magnetoencephalography laboratory at the Athinoula A. Martinos Center for Biomedical Imaging.
Research Consent Form (English / Spanish)
Research Assent Form (English / Spanish)
#1 Research Hospital in America
Mass General is the #1 research hospital in America, with a large percentage of that dedicated to Neuro-related diseases.
Support Neurosurgery
Philanthropic support for the Department of Neurosurgery at Mass General is critical to patient care, research and education.
