Press ReleaseJan | 17 | 2023
Modified CRISPR-Based Enzymes Improve the Prospect of Inserting Entire Genes into the Genome to Overcome Diverse Disease-Causing Mutations
Key Takeaways
- Investigators have developed an improved method to more accurately insert large DNA sequences–such as an entire normal replacement gene—in cells
- The advance may lead to the development of techniques that have wide-ranging clinical applications to fix diverse disease-causing mutations
BOSTON – Many genetic diseases are caused by diverse mutations spread across an entire gene, and designing genome editing approaches for each patient’s mutation would be impractical and costly.
Investigators at Massachusetts General Hospital (MGH) recently developed an optimized method that improves the accuracy of inserting large DNA segments into a genome.
This approach could be used to insert a whole normal or “wild-type” replacement gene, which could act as a blanket therapy for a disease irrespective of a patient’s particular mutation.
The work involves the optimization of a new class of technologies called CRISPR-associated transposases (CASTs), which are promising tools for large DNA insertions that can be easily targeted to a desired genomic site via a reprogrammable guide RNA.
However, in their natural state, CASTs have undesirable properties for genome editing applications—namely, suboptimal product purity (how often only the intended DNA sequence is inserted into the genome) and a relatively high rate of unwanted off-target integration at unintended sites in the genome.
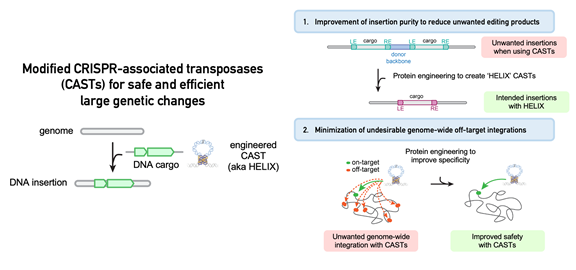
In their research published in Nature Biotechnology, a team led by first author Connor Tou, a graduate student at MIT and MGH, and senior author Ben Kleinstiver, PhD, an Assistant Investigator in the Center for Genomic Medicine at MGH and an Assistant Professor at Harvard Medical School, addressed these shortcomings by using protein engineering approaches to modify the properties of CAST systems.
They found that adding a certain enzyme called a nicking homing endonuclease to CASTs resulted in a dramatic increase in product purity towards the intended insertion.
Further optimization of CASTs’ structure led to DNA insertions with high integration efficiency at intended genomic targets with vastly reduced insertions at unwanted off-targets sites.
The researchers called the new and improved system “HELIX,” which is short for Homing Endonuclease-assisted Large-sequence Integrating CAST-compleX.
“We demonstrated a generalizable approach that can be used to modify a variety of CAST systems into safer and more effective versions that have high product purity and genome-wide specificity,” says Tou.
“By combining our insights, we created HELIX systems with greater than 96% on-target integration specificity—increased from approximately 50% for the naturally occurring wild-type CAST system. We also determined that HELIX maintains its advantageous properties in human cells.”
Kleinstiver notes that the technology could have applications beyond the ability to restore normal healthy genes to individuals with disease-causing mutations.
“Additionally, programmable DNA integration can facilitate cell engineering efforts where installation of large genetic sequences at targeted locations could endow cells with new capabilities while obviating safety, efficacy, and manufacturing issues resulting from traditional random integration approaches,” he says.
The study is also co-authored by Benno Orr.
This work was supported by the National Science Foundation and MGH.
About the Massachusetts General Hospital
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The Mass General Research Institute conducts the largest hospital-based research program in the nation, with annual research operations of more than $1 billion and comprises more than 9,500 researchers working across more than 30 institutes, centers and departments. In July 2022, Mass General was named #8 in the U.S. News & World Report list of "America’s Best Hospitals." MGH is a founding member of the Mass General Brigham healthcare system.
Type
Centers and Departments
Topics
Check out the Mass General Research Institute blog
Bench Press highlights the groundbreaking research and boundary-pushing scientists working to improve human health and fight disease.
Support Research at Mass General
Your gift helps fund groundbreaking research aimed at understanding, treating and preventing human disease.
