NewsNov | 6 | 2019
Closing the Genetic Gap in Orofacial Clefts
Facial structures such as our nose, lips and jaw bones are formed from cranial neural crest (CNC) cells, a group of pluripotent cells that delaminate from the neural tube during embryogenesis. Derangement of this process results in cleft lip and palate, the most common structural congenital malformations in humans. Researchers at Mass General for Children (MGfC) are looking into the specific genetic miscues leading to these craniofacial anomalies. Understanding the pathways and genetics involved in CNC malformation represents the next step forward in cleft diagnosis. A revolutionary advance would be to go beyond improved genetic diagnosis and identify targeted compounds to potentially mitigate orofacial clefts before they form, explains Chien-Wei (Eric) Liao, MD, PhD, who leads the Craniofacial Developmental Biology Laboratory at MGfC. Dr. Liao is also the co-director of the MGfC Cleft Lip and Craniofacial Clinic, a multidisciplinary clinic treating patients with craniofacial disorders.
Zebrafish Models of Human Clefts
To investigate the role specific pathways and genes may play in cleft pathogenesis, Dr. Liao developed a functional genomics model using the zebrafish, an animal sharing developmental similarities to human palate formation. Because zebra-fish develop quickly and are optically transparent at larval stages, researchers can observe structural anomalies as they develop. Using this model, Dr. Liao reproduced the pathology of a severe form of human orofacial cleft (oblique facial clefts) in the zebrafish (1). Specifically, he and his colleagues demonstrated that disrupting specc1lb, a homolog to the human SPECC1L gene known to cause clefts in humans, contributes to oblique facial clefts in zebrafish. This was the first time an animal model was used to mimic a human oblique facial cleft abnormality.
Cleft Formation in the Human Embryo and Zebrafish
Normal development and cleft formation in the human embryo at 10 weeks (left) and zebrafish embryo at 4 days post fertilization (DPF) (right). Studying the clefting similarities between the species offers insight into how clefting develops and ways to prevent or reverse the process early in development.

Compounds Involved in Orofacial Development
The team at MGfC also used chemical screens to map the development of CNC cells. Again using zebrafish, Dr. Liao found that when the animal was exposed to the nitric oxide synthase inhibitor 1-(2-[trifluoromethyl] phenyl) imidazole, or TRIM, it developed clefts as CNC maturation, migration and differentiation were impaired. This work proves that nitric oxide signaling and histone acetylation are necessary contributors that regulate CNC differentiation and convergence during development (2). The implications of this research are significant, as they point to the possible discovery of compounds that can be developed for pharmacologic manipulation of craniofacial development, in zebrafish and possibly in future human trials.
Chemically Induced Clefts
With exposure to the nitric oxide synthase inhibitor 1-(2-[trifluoromethyl] phenyl) imidazole, or TRIM, the zebrafish developed clefts (images on the right).
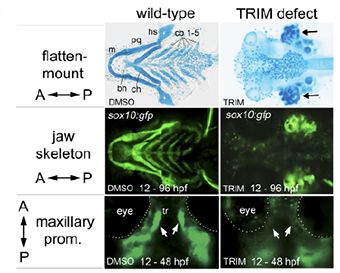
The lab continues to pursue this line of inquiry, focusing on the design of novel chemical screens to identify compounds that can actively disrupt abnormal CNC during development. The goal is to use the chemical screen in combination with the zebrafish model to produce a rapid, reliable model system of cleft malformations that will facilitate large-scale screens for drug discovery. With such a catalogue of chemical agents that are significant to this malformation, teams could work to uncover existing or new medicines that, when applied in utero, may halt or reverse cleft development.
Dr. Liao and his team are pursuing functional genomics research with funding support from the National Institutes of Health, which uses prenatal DNA sequencing to identify patients with congenital anomalies and to identify the genes involved in early human development. An end goal for the research would be for physicians to detect a predisposition to cleft in a baby’s DNA as early as six to eight weeks into a pregnancy, and to have effective pharmacological agents that promote proper palate development.
References
(1) Gfrerer L, V Shubinets, T Hoyos et al. “Functional analysis of SPECC1L in craniofacial development and oblique facial cleft pathogenesis.” Plastic and Reconstructive Surgery. 134, no. 4 (October 2014): 748-59.
(2) Kong Y, M Grimaldi, E Curtin et al. “Neural crest development and craniofacial morphogenesis are coordinated by nitric oxide and histone acetylation.” Chemistry & Biology. 21, no. 4 (April 2014): 488-501.

