NewsFeb | 3 | 2025
Research Spotlight: Innovative Nanovaccine Found to Trigger an Anti-Tumor Response for Rare Cancer
Hensin Tsao, MD, PhD, of the Department of Dermatology and Wellman Center for Photomedicine at Massachusetts General Hospital, is the senior author of a paper published in Bioactive Materials, “Fabrication and functional validation of a hybrid biomimetic nanovaccine (HBNV) against KitK641E-mutant melanoma.”
How would you summarize your study for a lay audience?
While we think of cutaneous melanoma as a sun-related skin cancer, those that develop on sun-protected sites, such as the palms of the hands, soles of the feet and fingernails are more rare but also more deadly. Moreover, these “acral” melanomas represent the most common types of melanoma diagnosed in patients with darker skin tones. Treating acral melanomas that have spread is extremely difficult as it is resistant to most current therapies. Previous research has revealed that acral melanomas exhibit more genetic alterations in the KIT oncogene compared to the other common forms of melanoma. Using this information, we developed a genetically engineered cell model of acral melanoma using the KIT gene. The advantage of this model is that we can test immune treatments in a mouse with an intact immune system.
To address the therapeutic challenge, we created an innovative hybrid biomimetic nanovaccine, which is designed to fight this type of melanoma by creating a “bionic” cell that has an outer layer of our mouse KIT melanoma wrapped around an inner nanoparticle. This vaccine uses advanced nanotechnology to teach the body’s immune system to recognize and destroy the cancer cells.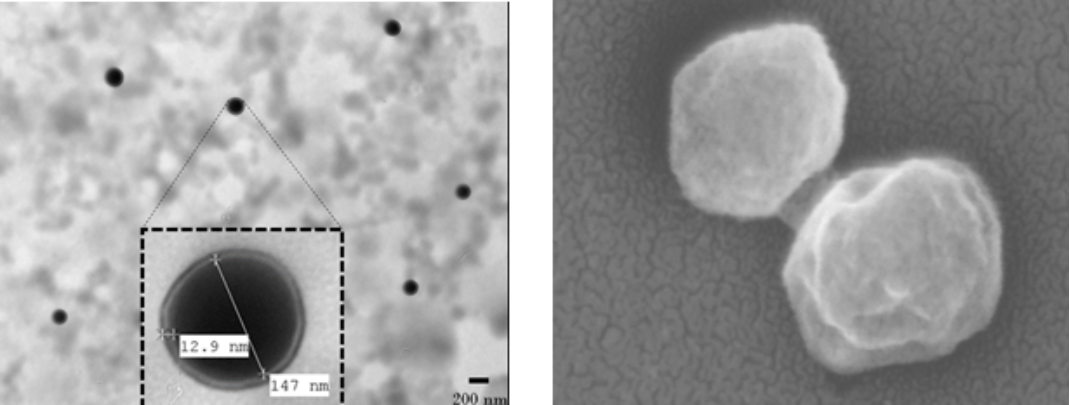
In a mouse model, the vaccine improved the body’s immune response, reduced tumor growth, and prevented KIT melanoma tumors from forming, if given as an early vaccine. This approach could be a potential, personalized therapy for other cancers that don’t respond to current treatments.
How do biomimetic nanovaccines work?
Nanovaccines deliver nanoparticles to enhance the immune system's ability to recognize and eliminate tumors. Traditional nanovaccines typically use synthetic nanoparticles, but biomimetic nanovaccines (BNVs) mimic natural biological structures. BNVs use natural cell membranes–such as those from tumor cells or immune cells–to coat nanoparticle cores. These hybrid designs help the vaccine interact more effectively with the body’s immune cells.
How did you apply biomimetic nanovaccines to cancer?
We were motivated to create a “hybrid” BNV (HBNV) using biomimetic nanotechnology to effectively treat KIT-mutated melanomas. The hybrid design combines cell membranes from our KIT-mutated melanoma and immune cells, called dendritic cells (DCs) from mice. As a nanoparticle, we were also able to put a “cargo” compound−imiquimod−that is known to further stimulate the immune system. We first tested the HBNV in cells in the lab to see if it could be effectively taken up by the proper immune cells. We then moved the studies into mice to see if the HBNV inhibits the growth of our KIT-mutated tumors.
What did you find?
What we found was absolutely fascinating. The HBNV was efficiently absorbed by both DCs and tumor cells. It helped DCs mature and made other immune cells more active, while decreasing the number of cells that weaken immunity.
In preclinical testing, the “fully-loaded” nanovaccine led to a dramatic reduction in tumor growth if the HBNV was administered after clinical evidence of tumor development. Interestingly, the nanovaccine also delayed or prevented tumors from developing if given before the tumor cells are injected into the mouse. Thus, the HBNV appears functional as both a therapeutic and preventative agent. Moreover, the vaccine targeted lymph nodes and tumors, suggesting that it could change the tumor environment to support immune responses. There were no observable toxic effects on the mouse.
What are the clinical implications?
These findings indicate that HBNVs are a promising strategy to treat KIT-mutated melanomas. Our work also provides a foundation for further development of HBNV approaches in cancer immunotherapy.
What are the next steps?
We would like this strategy pushed forwards toward the clinical setting, but we’ll have to conduct more research to assess and confirm the efficacy of the HBNV. We hope to one day apply for approval from the FDA to begin a clinical trial with patients with KIT-mutant cancers to test the vaccine’s safety, tolerability and immune response.
Authorship: In addition to Tsao, Mass General Brigham authors include Kishwor Poudel, Zhenyu Ji, Ching-Ni Njauw, Anpuchchelvi Rajadurai, Brijesh Bhayana, and Ryan J. Sullivan.
Paper cited: Poudel, K et al. “Fabrication and functional validation of a hybrid biomimetic nanovaccine (HBNV) against KitK641E-mutant melanoma” Bioactive Materials DOI: 10.1016/j.bioactmat.2024.12.023
-
![]()
- Clinical Director, MGH Melanoma& Pigmented Lesion Center
- Director, MGH Melanoma Genetics Program
Type
Centers and Departments
Check out the Mass General Research Institute blog
Bench Press highlights the groundbreaking research and boundary-pushing scientists working to improve human health and fight disease.
Support Research at Mass General
Your gift helps fund groundbreaking research aimed at understanding, treating and preventing human disease.
About Massachusetts General Hospital
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The Mass General Research Institute conducts the largest hospital-based research program in the nation, with annual research operations of more than $1 billion and comprises more than 9,500 researchers working across more than 30 institutes, centers and departments. MGH is a founding member of the Mass General Brigham healthcare system.

