Dou Laboratory: Zhixun Dou, PhD
Dou Laboratory
Zhixun Dou, PhD
Assistant Professor
Program Affiliations
Center for Regenerative Medicine
Krantz Family Center for Cancer Research
Explore This Lab
Overview

Aging is the greatest risk factor for cancer. The Dou laboratory aims to uncover the mechanisms of mammalian aging, with the ultimate goal of developing novel strategies to suppress aging and age-related diseases, including cancer. A major focus of the lab is chronic inflammation, a hallmark of aging and many chronic conditions. Inflammation in the absence of infection drives pain and tissue deterioration, promoting the progression of age-associated diseases. Our research investigates cellular senescence, autophagy, chromatin, and epigenetics in the context of aging and cancer. Through these studies, we aim to understand and target chronic inflammation to promote “healthy aging without diseases.”
Research Summary
Nuclear Degeneration in Aging and Cancer
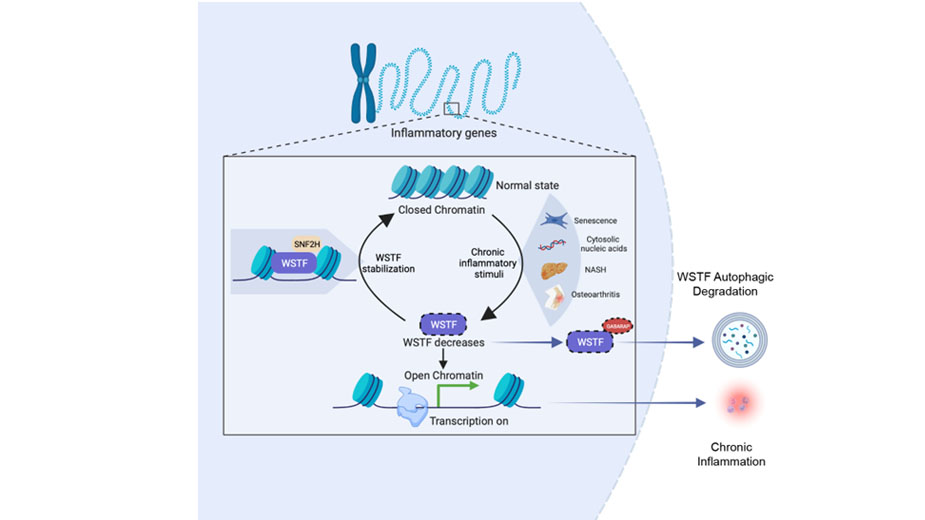
The nucleus is a central cellular entity. Studies within the Dou Laboratory suggests that the nucleus undergoes profound degeneration during aging and tumorigenesis. Nuclear components, including nuclear lamina and chromatin, can be degraded by autophagy in response to oncogene activation (Dou et al, Nature, 2015). While autophagy is generally viewed as a cytoplasmic recycling mechanism, the degradation of nuclear materials is beginning to unravel. Recently we reported the degradation of other nuclear substrates of autophagy, including SIRT1 (Xu et al, Nat Cell Biol, 2020) and WSTF of the ISWI chromatin remodeling complex (Wang et al, Nature, in press). Interestingly, WSTF degradation by autophagy specifically occurs under chronic and not acute inflammation. Restoring WSTF does not affect acute inflammation but suppresses chronic inflammation in cellular senescence as well as NASH and osteoarthritis in mouse models and patient samples. The ability to specifically target chronic inflammation without blunting acute inflammation offers a new approach for treating common chronic inflammatory diseases. Overall, we are interested in exploring novel nuclear substrates of autophagy and studying their significance in aging and cancer.
The Biology of Cellular Senescence
Cellular senescence, also referred to as the Hayflick limit, is a stable form of cell cycle arrest associated with pro-inflammatory responses. Senescence restricts proliferation of damaged cells and hence is a tumor suppressive mechanism. However, senescent cells accumulate in aged and diseased tissues, contributing to aging and many chronic diseases. Research in the Dou Laboratory shows that senescent cells generate chromatin fragments in the cytoplasm. This is interpreted by the cell as a “danger signal,” triggering the cytosolic DNA sensing cGAS-STING pathway and inflammation (Dou et al, Nature, 2017). The pro-inflammatory feature of senescence induces immuno-surveillance of oncogenic cells but contributes to age-associated diseases. Recently, we reported the upregulation of PD-L1 in senescence and aging (Onorati et al, Mol Cell Biol, 2020), as a consequence of chronic inflammation. This finding helps to explain why senescent cells are not effectively removed by the immune system during aging and chronic diseases. Furthermore, we discovered that chromatin fragments in the nucleus undergo nuclear egress, a membrane trafficking process at the nuclear envelope, to enter the cytoplasm, which is required to activate the cGAS-STING pathway and inflammation. Interestingly, nuclear egress is suppressed by AMPK and metformin (Kumazawa, et al, bioRxiv, 2024). Overall, we aim to unravel the genetic identity of cytoplasmic chromatin and to explore the mechanisms of its formation, with the goal to target it to block chronic inflammation associated with senescence and aging. We are also broadly interested in the biology of senescence beyond aging and cancer.
We are a founding member of the NIH SenNet Program aiming to comprehensively identify and characterize the differences in senescent cells across the body, across various states of human health, and across the lifespan. We are also part of the Technology Development and Application (TDA) Projects, employing single-cell proteomic technology to investigate senescent cells, using the lung as a model system. Our team contributed to several review papers from the consortium, improved the single-cell proteomic platform, deposited several single-cell datasets of mouse lungs, and made new discoveries in the lung using single-cell technologies.
Research Image
Roles of WSTF, a chromatin remodeling complex, in regulating chronic inflammation. Under chronic inflammatory stimuli, including senescence, chronic cytosolic nucleic acid exposure, NASH, and osteoarthritis, WSTF is downregulated by nuclear autophagy via a direct interaction with GABARAP. Consequently, loss of WSTF results in increased chromatin accessibility and transcription of inflammatory genes. By stabilizing WSTF protein levels through overexpression or utilizing a peptide that disrupts the WSTF-GABARAP interaction, chromatin accessibility over inflammatory genes is inhibited, suppressing chronic inflammation. Notably, WSTF degradation is not observed under acute inflammation, offering a mechanistic separation of chronic and acute inflammation.
Publications
Selected Publications
Wang Y, Eapen VV, Liang Y, Kournoutis A, Sherman M, Xu Y, Onorati A, Li X, Zhou X, Corey K, Du K, Burkard AC, Ho CK, Xie J, Zhang H, Díaz R, Ma X, Rieprecht U, O’Brien T, Cetinbas M, Wang L, Liu J, Bretz C, Havas A, Zhou Z, Sui SH, Saladi SV, Sadreyev R, Adams PD, Kingston RE, Diehl AM, Alman B, Goessling W, Yue Z, Wang XF*, Johansen T*, Dou Z*. WSTF nuclear autophagy regulates chronic but not acute inflammation. Nature. In press.
Kumazawa T, Xu Y, O’Brien TC, Lee JW, Wang Y, Cetinbas M, Sadreyev RI, El-Bardeesy N, Cheng CW, He B, Dou Z. Metformin inhibits nuclear egress of chromatin fragments in senescence and aging. bioRxiv. 2024.
Onorati A, Havas AP, Lin B, Rajagopal J, Sen P, Peter D. Adams PD, Dou Z. Upregulation of PD-L1 in senescence and aging. Mol Cell Biol. 2022 Oct 20;42(10):e0017122.
Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J, Lu C, Nicastri M, Bretz C, Winkler JD, Amaravadi R, Garcia BA, Adams PD, Ott M, Tong W, Johansen T, Dou Z*, Berger SL.* SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020 Oct; 22(10):1170-1179.
Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z, Capell BC, Xu C, Xu M, Kieckhaefer JE, Jiang T, Shoshkes-Carmel M, Tanim KMAA, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017. Oct 19;550(7676):402-406.
Dou Z, Xu C, Donahue G, Shimi T, Pan J-A, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, Catanzaro JM, Ricketts MD, Lamark T, Adam SA, Marmorstein R, Zong W-X, Johansen T, Goldman RD, Adams PD, Berger SL. Autophagy mediates degradation of nuclear lamina. Nature. 2015 Nov 5;527(7576):105-9.
* co-corresponding authors
Lab Members
Zhixun Dou, PhD
Dou laboratory:
Ana Maria Cabral Burkard
Zhixun Dou, PhD
Chia-Kang Ho, MD, PhD
Sovannarith Korm, PhD
Ji-Won Lee, PhD
Tara O’Brien
Jun Bum Park, PhD
Yanxin Xu, PhD
Yang Yang, PhD
Yu Wang, PhD
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
