Scadden Laboratory: David Scadden, MD
Email: dscadden@mgh.harvard.edu
David T. Scadden, MD
Gerald and Darlene Jordan Professor of Medicine, Harvard University
Professor, Krantz Family Center for Cancer Research
Director, Center for Regenerative Medicine, Massachusetts General Hospital
Professor of Stem Cell and Regenerative Biology, Harvard University
Co-Director, Harvard Stem Cell Institute, Harvard University
Explore This Lab
Overview

The Scadden Lab focuses on blood and bone marrow. Our goals are to define what governs blood cell production, how those processes are corrupted in disease, and how that information can be leveraged to develop novel therapies. We emphasize molecular, genetic, computational, and high-resolution imaging techniques to define how single molecules and single cells contribute to homeostasis, the success of stem cell transplantation, and the development of cancer. We emphasize those projects that can teach fundamental principles of biology while pointing the way to clinical advances.
Research Summary
It is increasingly evident that both bone marrow stroma and the hematopoietic stem cells (HSCs) they support comprise a heterogeneous mix of clones, each with defined functional traits. Stromal stem cells that form niches and HSCs are distinct and have limited plasticity, constrained by epigenetic features set early in development. These features persist under physiological stress, leading to selective expansion of specific clones. As a result, hematopoietic tissue begins with a diverse mix of stem cells that becomes progressively constrained through the stresses of aging and environmental challenges. These stresses, including random mutations, affect different clones in distinct ways. Additionally, the interaction between stromal and hematopoietic clones in vivo further diversifies clonal outcomes. Thus, hematopoiesis serves as an excellent model for studying clonal dynamics and their impact on tissue behavior, resilience, and vulnerability.
We apply this framework to study several disease states:
1. Selective outgrowth of pathogenic inflammatory clones:
Clonal hematopoiesis, characterized by overgrowth of HSCs with specific mutations, is linked not only to malignancy but also to chronic conditions such as cardiac, lung, liver, kidney diseases, and osteoporosis. We suggest that mutations mark clonal selection, which more broadly reflects selection based on functional traits like cytokine sensitivity. Chronic conditions such as hypertension or obesity—and even temporary states like pregnancy—may select for particular HSC subpopulations. These positively selected populations establish a new homeostatic baseline that retains a “memory” of prior stress, shaping future responses. We term this concept clonal hematopoiesis of epigenetic provenance (CHEP), which is currently under investigation.
2. Selective HSC-stroma interactions supporting malignancy:
Our prior work demonstrated that leukemic clones alter bone marrow stromal composition. Additionally, acquired genetic abnormalities in stroma alone can induce myelodysplastic syndromes (MDS) and the expansion of malignant clones. We hypothesize that a bidirectional selection between hematopoietic and stromal clones promotes malignancy. Ongoing studies aim to identify the molecular mediators of this intercellular cooperation. Pinpointing these modifiers may reveal new therapeutic targets to disrupt disease-promoting interactions.
3. Transient vulnerability to eliminate chemotherapy persistence:
Exogenous exposures reshape clonal composition, and we propose the same holds true for leukemia under chemotherapy. We tested whether chemotherapy selects for leukemic cells with metabolic features that enable survival during the stress of treatment. Using untargeted metabolomics in vivo, we profiled acute myeloid leukemia (AML) cells before, immediately after, and during relapse. Post-chemotherapy cells showed distinct metabolic traits not seen during relapse or detected via genetics. Persistence-related pathways were identified using small molecule and CRISPRi screens. We validated several gene products critical to this persistent state and showed that inhibiting them post-chemotherapy improved survival and reduced relapse in humanized mouse models of chemoresistant AML. Drug development efforts targeting these vulnerabilities are now underway.
4. Thymus niche manipulation to enhance adaptive immunity:
Building on earlier identification of HSC niche components in bone marrow, we examined the thymus, essential for α/β T cell development and adaptive immunity. Though long thought to be vestigial in adults, we recently showed that the thymus remains vital to human health; thymectomy in middle age increases 10-year mortality, primarily due to cancer. Others have demonstrated thymus regeneration via lymphoid progenitor infusion. We have now identified a mesenchymal cell subset in the thymus that acts as a niche, recruiting circulating T-competent progenitors. Furthermore, we found that some HSC subclones exhibit enhanced T cell potential, a trait that persists through serial transplantation. We are currently developing strategies for thymic regeneration by simultaneously enhancing niche function and HSC T cell competence, aiming to restore immune function in aging.
Research Image
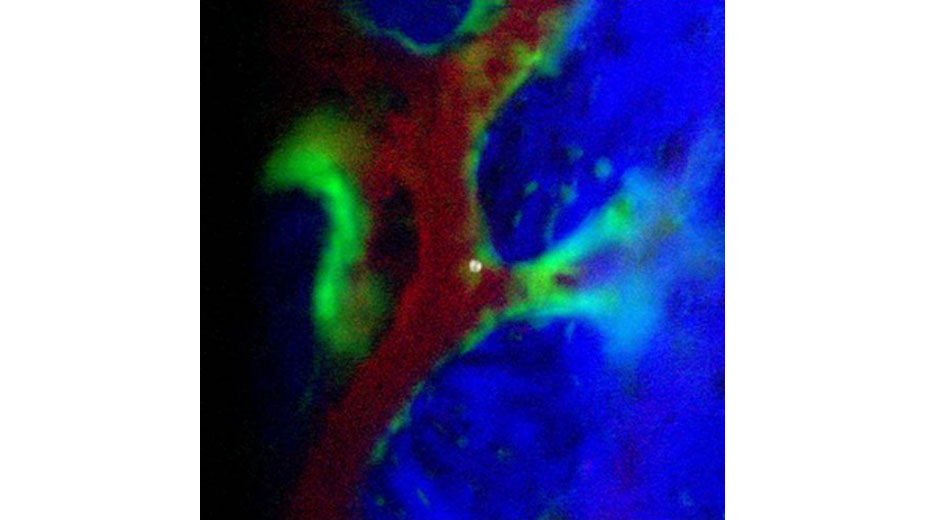
We are interested in physiology resolved to single cell and single molecule events. How a tissue responds to stress is ultimately conducted at cell and molecular levels that offer opportunities for manipulation to improve resilience. Below is a high resolution in vivo image obtained in collaboration with Dr. Charles Lin that captures a single hematopoietic stem cell after transplantation as the engraftment process begins.
Lab Members
David T. Scadden, MD
Scadden Laboratory:
Lei Chen, PhD
Ray (Yu-Hao) Cheng, MD, PhD
Karin Gustafsson, PhD
Kameron Kooshesh, MD
Trine Ahn Kristiansen, PhD
Dan Li, PhD
Christina Mayerhofer, MD
David T. Scadden, MD
Azeem Sharda, MSc
Jun Xia, PhD
Ting Zhao, PhD
Publications
Selected Publications
Kooshesh K, Foy BH, Sykes DB, Gustafsson KU, Scadden DT. The adult thymus is critical for health. N Engl J Med. 2023;389:406-417.
Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, Dionne D, Papazian A, Lee D, Ashenberg O, Subramanian A, Vaishnav ED, Rozenblatt-Rosen O, Regev A, Scadden DT. A cellular taxonomy of the bone marrow stroma in hematopoiesis and leukemia. Cell. 2019 Jun 13;177:1915.
Yu V, Yusuf RZ, Oki T, Wu J, Saez B, Wang X, Cook C, Baryawno N, Ziller MJ, Lee E, Gu H, Meissner A, Lin CP, Kharchenko PV, Scadden DT. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell. 2016 Nov 17;167(5):1310-1322.
Raaijmakers MHGP, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor cell dysfunction induces myelodysplasia enabling secondary leukemia. Nature. 2010;464(7290):852-857.
Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92-96.
Calvi LM, Adams GB, Kronenberg H, Scadden DT. Osteoblastic cells regulate the hematopoietic stem cell niche. Nature. 2003;425(6960):841-846.
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
Learn More
Learn more about our work at our main lab website.
