The Thoracic Oncology Laboratory of Michael Lanuti, MD
Contact Information
The Thoracic Oncology Laboratory of Michael Lanuti, MD
55 Fruit Street
Boston,
MA
02114
Email: mlanuti@mgh.harvard.edu
Michael Lanuti, MD
Director, Thoracic Oncology, Division of Thoracic Surgery, Massachusetts General Hospital
Associate Professor, Harvard Medical School
Explore This Lab
About the Lab
The Thoracic Oncology Laboratory of Michael Lanuti, MD, in the Division of Thoracic Surgery at Massachusetts General Hospital spearheads translational research focused on thoracic oncology. The principal goals of the laboratory are to design novel therapeutics to treat lung and esophageal cancer to bring to clinical trials. The laboratory uses oncolytic viruses in combination with other modalities, such as chemotherapy and angiotensin system inhibitors, to target solid tumors. Some of these strategies include development of oncolytic viruses that help degrade tumor matrix.
The largest area of research is the investigation of losartan and lisinopril in the multi-modality management of lung or esophageal cancer in animal models. In non-small cell lung cancer, the laboratory has demonstrated an improved antitumor effect in the presence of chemotherapy. It is investigating whether this phenomenon can be observed with radiation in an in vitro model and in vivo model.
Another lab interest is molecular imaging of pulmonary fibrosis and radiation induced lung injury. Dr. Lanuti and collaborators have pioneered the first positron emission tomography (PET) probe with high affinity to collagen 1 to be used in the quantitation of patients carrying a diagnosis of pulmonary fibrosis or radiation induced lung injury. He has an NIH funded human clinical trial to better characterize radiation-induced lung injury, which may ultimately be used to help differentiate lung injury from tumor recurrence.
Research Projects
PET Imaging of Radiation Induced Lung Injury
- Dates: April 2020-2023
- Funding: NIH/NHLBI 1R44CA250771-01
- Description: The goal of the proposed research is to optimize a type I collagen-specific PET probe 68Ga-CBP8 for direct imaging of radiation induced lung injury
PET Imaging of Pulmonary Fibrosis
- Dates: April 2016 to March 2021
- Funding: NIH/NHLBI R01 HL 131907-01
- Description: The goal of the proposed research is to implement a type I collagen-specific PET probe for direct imaging of pulmonary fibrosis
Noninvasive Quantification of Pulmonary Fibrosis Due to Radiation Induced Lung Injury Using [68Ga]CBP8 Type 1 Collagen Probe
- Dates: October 2019-2021
- Funding: Chest Foundation Grant
The Boston Lung Cancer Survival Cohort
- Dates: June 2017 to May 2022
- Funding: NIH/NCI 5U01 CA209414-03
- Description: This study uses the combination of biomarker data, tumor characterization, traditional epidemiologic risk factor data and electronic medical records to facilitate powerful translational research and provide unique opportunities to explore predictors of overall survival and treatment outcomes
Ananta Pharmaceuticals Sponsored Research Agreement
- Dates: April 2018 to October 2020
- Description: This collaboration examines the effects of a cyclophilin inhibitor EDP-494 and an FXR agonist EDP-305 on fibrosis development in the murine bleomycin model
Notable Contributions and Publications
**indicate publications by trainees while in the lab
Investigating Molecular Markers and Oncogenes to Predict Recurrence in Resected Early-Stage Lung Cancer
Dr. Lanuti has clinical research interests in the predictive value of oncogenes in resected stage I non-small cell lung cancer (NSCLC). His team has published on the prognostic significance of EGFR and KRAS mutation status in treatment naive, surgically resected stage I NSCLC which was predictive of improved outcome. Patients harboring KRAS mutations appeared to have worse outcomes and may be considered for adjuvant therapy. The lab has also combined the detection of oncogene driver mutations coupled with predictive gene signatures to further delineate a high-risk cohort of patients with resected stage I NSCLC.
Related Publications
- Izar B**, Zhou H**, Heist RS, Azzoli CG, Scriber EEF, Muzikansky A, Bernardo L, Dias-Santagata D, Iafrate AJ, Lanuti M. The Prognostic Impact of KRAS Mutation on Survival in Resected Stage I Lung Adenocarcinoma. J Thor Oncol 2014 Sep. 9(9):1363-9. doi: 10.1097/JTO.0000000000000266
- Izar B**, Sequist L, Lee M, Muzikansky A, Heist RS, Iafrate AJ, Dias-Santagata D, Mathisen DJ, Lanuti M. Impact of EGFR Mutation Status on Outcomes in Patients with Resected Stage I NSLC. Ann Thorac Surg. 2013 Sep. 96(3):962-8. doi: 10.1016/j.athoracsur.2013.05.091
- Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS, Lennes IT, Digumarthy S, Waltman BA, Bast E, Tammireddy S, Morrissey L, Muzikansky A, Goldberg SB, Gainor J, Channick CL, Wain JC, Gaissert H, Donahue DM, Muniappan A, Wright C, Willers H, Mathisen DJ, Choi NC, Baselga J, Lynch TJ, Ellisen LW, Mino-Kenudson M, Lanuti M, Borger DR, Iafrate AJ, Engelman JA, Dias-Santagata D. Implementing Multiplexed Genotyping of NCSLC into Routine Clinical Practice. Ann Oncol. 2011 Dec. 22(12):2616-24. doi: 10.1093/annonc/mdr489
Angiotensin System Inhibitors Are Associated with Improved Survival in Locally Advanced NSCLC and Exhibit Anti-Tumor Synergism with Chemotherapy in a Murine Model of NSCLC
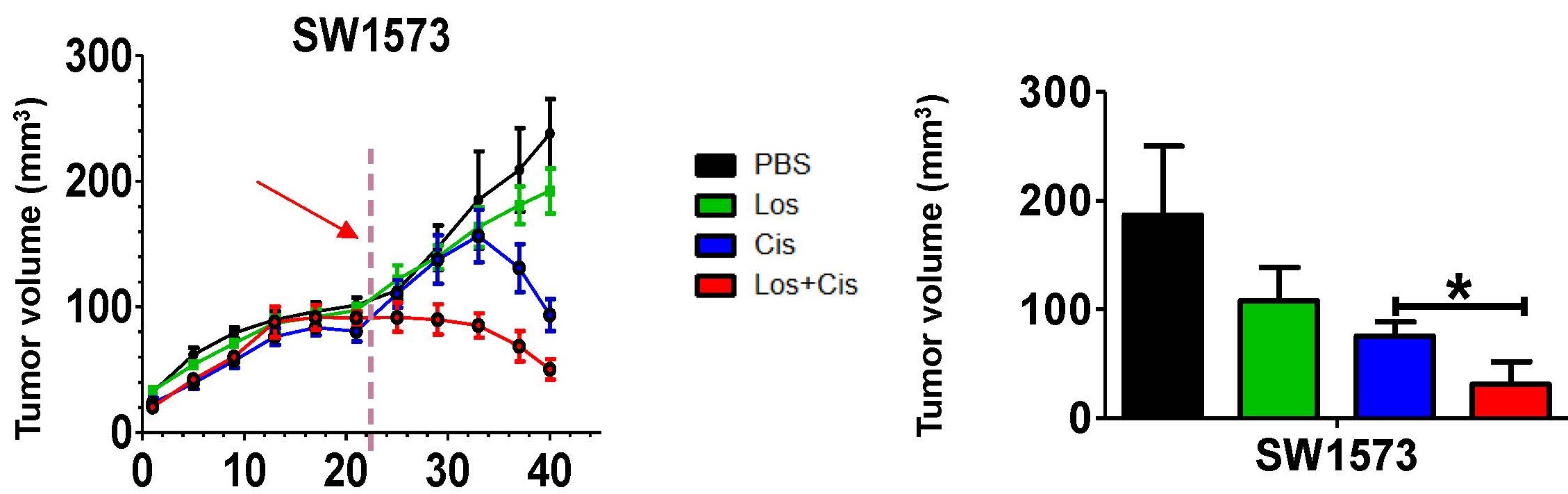
One of the major focuses of the lab is exploring the anti-tumor effect of angiotensin converting enzyme (ACE) and angiotensin receptor blocker (ARB) inhibitors on lung and esophageal cancer. It found that angiotensin system inhibitors are associated with improved patient survival in stage III NSCLC. It also demonstrated enhanced cytotoxic effect of cisplatin in lung cancer in animal models. The mechanism appears to be related to epithelial to mesenchymal transition of tumor.
Related Publications
- Tang H**, Sojoodi M, Li S**, Erstad DJ**, Ghoshal S, Aror G, Abston E, Tanabe KK, Fuchs BC, Lanuti M. Angiotensin System Inhibitors are Associated with Improved Survival in the Multi-Modality Treatment of Stage IIIA NSCLC and Have a Significant Anti-Tumor Effect in Combination with Chemotherapy in a Murine Model of Lung Cancer. Abstract presented at IASLC 2019 World Conference on Lung Cancer, Barcelona, Spain
- Tang H**, Sojoodi M, Wang Y, Wei Y, Li S**, Erstad DJ**, Ghoshal S, Arora G, Abston E, Tanabe KK, Fuchs BC, and Lanuti M. Angiotensin System Inhibitors are Associated with Improved Survival in Locally Advanced NSCLC and Exhibit Anti-Tumor Synergism with Chemotherapy in a Murine Model of NSCLC. Submitted to Clinical Cancer Research
Molecular Imaging of Pulmonary Fibrosis and Radiation-Induced Lung Injury with a Novel PET Probe with High Affinity to Collagen I
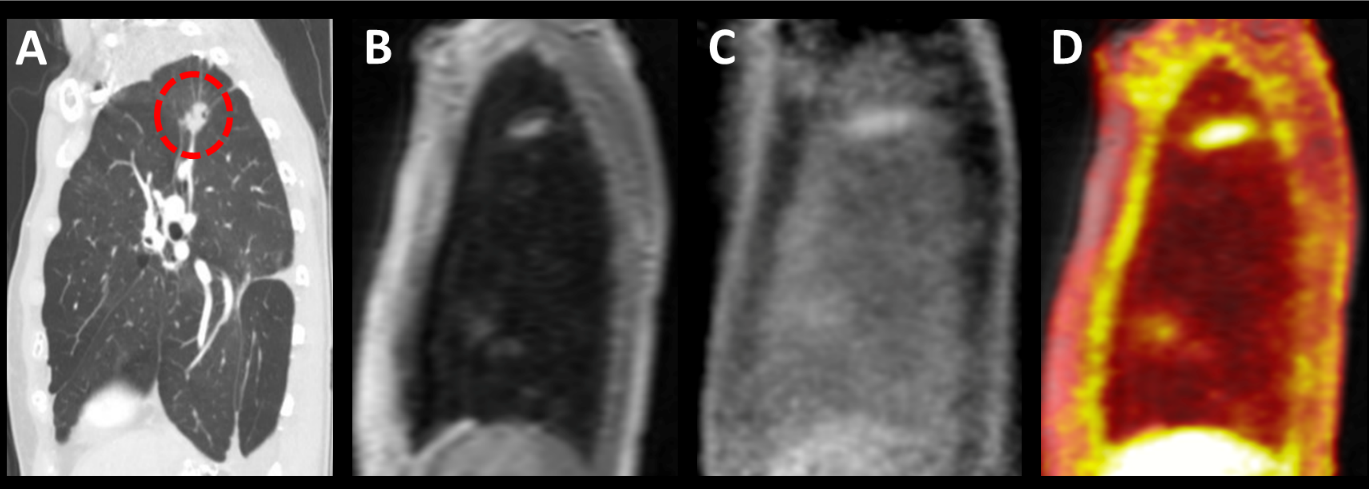
Dr. Lanuti has been collaborating with the Martino Center of Molecular Imaging at Mass General in the study of specific molecular probes. He currently has NIH funding to study a collagen I PET probe in humans as an extension of a line of investigation that demonstrated the detection of pulmonary fibrosis in an animal model that has also be tested in human subjects. The PET probe can detect patients with pulmonary fibrosis and may be more sensitive than CT chest in demonstrating progression. The team has now embarked on studying this PET probe in radiation induced lung injury and to help distinguish radiation injury from tumor if combined with FDG PET.
Related Publications
- Caravan P, Fuchs BC, Zachariah R**, Mino-Kenudson M, Lanuti M. Molecular MR Imaging of Pulmonary Fibrosis in Mice. Amer J Resp Cell and Mol Bio 2013; 49(6), pp 1120–1126
- Désogère P**, Tapias LF**, Hariri LP, Rotile N, Rietz TA, Probst CK, Blasi F, Day H, Elliot J**, Mino-Kenudson M, Weinreb P, Violette SM, Tager AM, Lanuti M, Caravan P. Type I Collagen-Targeted PET Probe for Pulmonary Fibrosis Detection and Staging in Preclinical Models. Science Trans Med. 2017 Apr 5. 9(384). doi: 10.1126/scitranslmed.aaf4696
- Désogère P, Tapias LF**, Rietz TA, Rotile N, Blasi F, Day H, Elliot J**, Fuchs BC, Lanuti M, Caravan P. Optimization of a Collagen-Targeted Positron Emission Tomography Probe for Molecular Imaging of Pulmonary Fibrosis. J Nucl Med. 2017 Dec. 58(12):1991-1996. doi: 10.2967/jnumed.117.193532
- Montesi SB, Izquierdo-Garcia D, Désogère P**, Abston E**, Liang LL, Digumarthy S, Seethamraju R, Lanuti M, Caravan P, Catana C. Type I Collagen-Targeted PET Imaging in Idiopathic Pulmonary Fibrosis: First-in-Human Studies. Am J Respir Crit Care Med. 2019 Jul 15;200(2):258-261
- Akam EA, Abston E**, Rotile NJ, Slattery HR, Zhou IY, Lanuti M, Caravan P. Improving the Reactivity of Hydrazine-bearing MRI Probes for in vivo Imaging of Lung Fibrogenesis. Chem Sci. 2019 Nov 8. 11(1):224-231. doi: 10.1164/rccm.201903-0503LE
About Michael Lanuti, MD

After receiving a Bachelor of Science in Bioengineering from the University of Pennsylvania, Michael Lanuti, MD, received his medical degree from the University of Pennsylvania School of Medicine. He completed his internship and residency in surgery at the Hospital of the University of Pennsylvania and a two-year research fellowship in a thoracic oncology laboratory focusing on novel treatments (including oncolytic virus) for lung cancer.
Dr. Lanuti continued with sub-specialty training and completed a cardiothoracic fellowship at Mass General. He has been on staff in the Division of Thoracic Surgery since 2004 and holds a parallel appointment as associate professor of surgery at Harvard Medical School (HMS). He has been the Friedman-Lambert Scholar in Academic Thoracic Surgery at Mass General/HMS since 2004. He is the director of thoracic oncology and the thoracic surgery liaison to the Mass General Brigham Cancer Institute.
His clinical interests include minimally invasive surgery for lung cancer, complex airway tumors, multimodality treatment of esophageal cancer, advanced staging procedures, navigation bronchoscopy and thermal ablation of lung tumors.
Multimedia
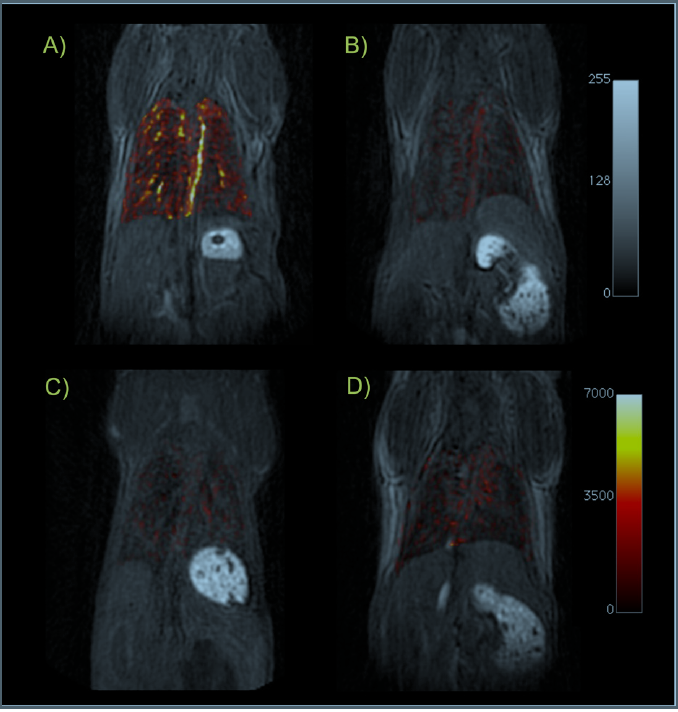
Ultrashort echo time (UTE)-MRI of the lungs. Coronal T1-weighted UTE images of mice from each cohort in this study: (A) BM mouse + collagen-targeted EP-3533; (B) sham mouse + EP-3533; (C) BM + control probe EP-3612; (D) sham + EP-3612. False color overlay is the difference image obtained by subtracting the UTE image acquired before probe injection from the UTE image taken after probe injection. Mice with pulmonary fibrosis that receive the collagen-specific probe (A) show much higher pulmonary signal enhancement than sham mice or mice receiving the control probe. All images rendered at the same scale.
A Top Hospital in America
Mass General is recognized as a top hospital on the U.S. News Best Hospitals Honor Roll for 2025-2026.
Michael Lanuti, MD, on Advances in Motion
Advances in Motion highlights the latest breakthroughs, research and clinical trials from Mass General.
Numerous Milestones in Thoracic Surgery
For more than 70 years, patients from around the world have come to the Mass General Division of Thoracic Surgery for surgical care.
