Krantz Family Center for Cancer Research
Blum Laboratory
Contact Information
Blum Laboratory
(opens fall 2025)
Steven M. Blum, MD
Faculty Member
Mass General Brigham Cancer Institute
Harvard Medical School
Program Affiliations
Krantz Family Center for Cancer Research
Explore the Blum Lab
Overview

Recent advances in cancer immunotherapy have delivered life-saving benefits to patients with many types of cancer. However, most tumors do not respond to current treatments, especially when the cancer has spread to certain parts of the body. These therapies can also cause serious side effects called immune-related adverse events (irAEs), which happen when the immune system attacks healthy organs.
The Blum Laboratory works to make cancer immunotherapy both safer and more effective. We study patient samples and use advanced techniques to find new treatment strategies. Our early work has focused on two main challenges: (1) understanding irAEs and how they relate to the cancer-fighting effects of immunotherapy and (2) developing better treatments for cancers that spread to the abdomen and cause a condition called malignant ascites.
Our long-term goal is to improve the lives of cancer patients by boosting anti-tumor immunity while reducing the harm caused by treatments.
Research Summary
Immune checkpoint inhibitors (ICIs) have transformed cancer therapy and can produce durable responses in some patients with solid tumors. Unfortunately, most patients do not benefit from these treatments. Many tumors are resistant to ICIs, particularly when cancer cells have spread to certain anatomical sites. Additionally, ICI use can be complicated by immune-related adverse events (irAEs), which occur when the immune system attacks healthy organs. IrAEs can lead to the discontinuation of effective therapies, hospitalizations, permanent disability, or even death.
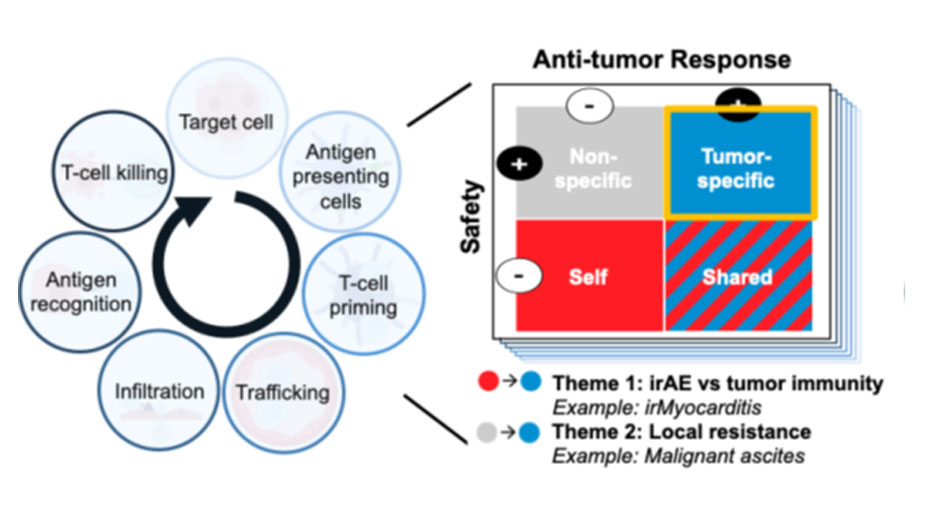
The Blum Laboratory aims to develop safer and more effective immunotherapy strategies for patients with solid tumors. Using patient samples, systems immunology, and disease-relevant model systems, we investigate the cellular and molecular drivers of toxicity and treatment resistance across the tumor immunity cycle (see Figure). Our long-term goal is to uncover broadly applicable principles that can improve clinical outcomes by (Theme 1) mitigating irAEs while preserving anti-tumor immunity and (Theme 2) overcoming local immunotherapy resistance. The examples below illustrate current projects within these broader themes.
Theme 1: Investigating irAEs and Anti-Tumor Immunity through irMyocarditis
IrAEs can affect any organ system and have clinical manifestations that range in severity from mild to life-threatening. Current treatments for severe irAEs rely on broadly immunosuppressive medications, which can compromise tumor control and may not optimally treat the mechanisms driving the organ-specific irAE.
Our first efforts to explore the relationship between irAEs and anti-tumor immunity have focused on ICI-related myocarditis (irMyocarditis), a particularly dangerous irAE that occurs in ~1% of ICI recipients but is frequently fatal. We used single-cell RNA sequencing, T-cell receptor (TCR) sequencing, multiplexed imaging, and TCR functional assays to map the immune responses associated with irMyocarditis onset and severity. These studies have revealed potentially pathogenic pathways in both immune and non-immune cells that could be targeted by existing drugs not yet used for this condition. We also found that T-cell responses in the heart differ from those in the tumor, raising the possibility that anti-tumor immunity and irAEs could be therapeutically separated. Building on these findings, we are developing cutting-edge models to identify mechanistic drivers of irMyocarditis and test targeted interventions. While irMyocarditis serves as an important example for understanding irAEs and anti-tumor responses, our larger program is applying this framework to understand and manage irAEs affecting all organ systems.
Theme 2: Improving Immune Responses in Patients with Malignant Ascites
The spread of cancer to the peritoneal cavity can lead to the development of malignant ascites, a condition where fluid accumulates in the abdomen due to cancer. Malignant ascites occurs in ~8% of all cancer patients and up to 40% of those with gastroesophageal adenocarcinoma (GEA). Ascites is associated with severe symptoms and poor response to systemic therapies, including ICIs. The immunologic barriers in this unique microenvironment remain incompletely understood.
Our initial studies have focused on GEA-associated ascites. Using paired single-cell transcriptomic and surface proteomic profiling of more than 500,000 immune and tumor cells from ascites and matched blood, combined with proteomic analysis of ascites fluid and plasma, we have identified:
- A dendritic cell subset enriched in malignant ascites across multiple tumor types that is anticipated to have an immunosuppressive function in the tumor microenvironment.
- Targetable soluble proteins and novel immune checkpoints concentrated in the peritoneal microenvironment.
We are now using patient-derived ex vivo models to dissect the biology of these targets and test therapeutic strategies. Ultimately, these insights aim to guide the development of approaches that would overcome local immunosuppression while minimizing systemic toxicity. Such strategies could benefit a wide range of cancers that metastasize to body cavities through either systemic or intraperitoneal delivery.
Research Image
Model for improving outcomes for cancer patients receiving immunotherapy. Each step of the tumor immunity cycle (adapted from Mellman I, et al. Immunity 2023.) presents an opportunity to identify safe, tumor-specific mechanisms that can help to improve both the safety and efficacy of cancer immunotherapy. The lab’s main research themes of (Theme 1) improving safety without compromising efficacy and (Theme 2) safely overcoming local immunotherapy resistance are shown with example projects.
Select Publications
Blum SM*, Ouyang B*, Zubiri L, Leonard D, Slowikowski K, Wang M, Grealish KA, Hathaway NK, Molina G, Shah N, Lawrence DP, Dougan M, Villani AC, Mino Kendusen M, Reynolds KL**, Sullivan RJ**. Tumor location as a risk factor for severe immune-related adverse events. J Immunother Cancer. 2025 May 15;13(5):e011312.
Blum SM*, Zlotoff DA*, Smith NP*, Kernin IJ*, Ramesh S*, Zubiri L, Caplin J, Samanta N, Martin S, Wang M, Tirard A, Song Y, Xu KH, Barth J, Sen P, Slowikowski K, Tantivit J, Manakongtreecheep K, Arnold BY, Nasrallah M, Pinto CJ, McLoughlin D, Jackson M, Chan P, Lawless A, Michaud WA, Sharova T, Nieman LT, Gainor JF, Wu CJ, Juric D, Mino-Kenudson M, Oliveira G, Sullivan RJ, Boland GM, Stone JR, Thomas MF**, Neilan TG**, Reynolds KL**, Villani AC**. Immune responses in checkpoint myocarditis across heart, blood and tumour. Nature. 2024 Dec;636(8041):215-223.
Zhao JJ*, Ong CJ*, Srivastava S, Chia DKA, Ma H, Huang K, Sheng T, Ramnarayanan K, Ong X, Tay ST, Hagihara T, Tan ALK, Teo MCC, Tan QX, Ng G, Tan JW, Ng MCH, Gwee YX, Walsh R, Law JH, Shabbir A, Kim G, Tay Y, Her Z, Leoncini G, Teh BT, Hong JH, Tay RYK, Teo CB, Dings MPG, Bijlsma M, Lum JHY, Mathur S, Pietrantonio F, Blum SM, van Laarhoven H, Klempner SJ, Yong WP, So JBY, Chen Q**, Tan P**, Sundar R**. Spatially Resolved Niche and Tumor Microenvironmental Alterations in Gastric Cancer Peritoneal Metastases. Gastroenterology. 2024 Dec;167(7):1384-1398.e4.
Blum SM, Rouhani SJ, Sullivan RJ. Effects of immune-related adverse events (irAEs) and their treatment on antitumor immune responses. Immunol Rev. 2023 Sep;318(1):167-178.
*Denotes co-first authorship
**Denotes co-senior authorship
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
Support the Krantz Family Center for Cancer Research
When you support us you are enabling discoveries that will lead to effective new weapons in the battle against cancer.
