Krantz Family Center for Cancer Research
Mount Laboratory
Contact Information
Mount Laboratory
(opens winter 2026)
Christopher W. Mount, MD, PhD
Faculty Member
Mass General Brigham Cancer Institute
Harvard Medical School
Program Affiliations
Krantz Family Center for Cancer Research
Explore the Mount Lab
Overview

The Mount laboratory uses single cell technologies to unravel the interactions between cell therapies and the glioma microenvironment. We leverage these fundamental insights to develop enhanced cell therapies for adult and pediatric glioma patients. Gliomas are the most common primary brain tumors and frequently have a devastatingly poor prognosis and few treatment options. Developing novel therapies for these tumors poses unique challenges, including extensive tumor heterogeneity, limited penetration of many targeted therapies into the brain, and difficulties in developing model systems of these diseases. Engineered cellular immunotherapies, in which a patient’s immune cells are altered to fight tumor cells, may be capable of overcoming many of these limitations. In collaboration with colleagues across neuro-oncology, neurosurgery, and neuropathology, we champion a ‘bedside to bench to bedside’ philosophy that leverages primary human samples to inform development of faithful model systems to assess these therapeutics in the laboratory and evaluate their activity in clinical trials.
Research Summary
Gliomas are the most common and deadly brain tumors occurring in adults. Despite decades of concerted efforts, outcomes in this disease remains dismal, and there is a desperate need for improved therapies. While advances in immunotherapy have revolutionized patient care and achieved transformative benefits in other realms of oncology, these impacts have yet to be realized in glioma patients. Chimeric antigen receptor (CAR)-T cell therapies, in which engineered molecules encoding extracellular tumor-targeting domains are paired with intracellular T cell activation domains, have recently shown promising but heterogeneous results in early phase clinical trials for glioma patients. The transcriptional heterogeneity of gliomas across patient populations and within individual tumors is thought to be a major obstacle to these therapies, and emerging large single cell sequencing datasets of molecularly-defined glioma cohorts offer the potential to refine target selection strategies for adoptive cell therapies in these diseases. Evolving spatial multiomic platforms additionally provide the opportunity to understand this heterogeneity in the context of the complex glioma microenvironment, and the potential to uncover cellular mediators of resistance in situ. Integrating these approaches in faithful model systems of glioma-cell therapy interactions will allow us to probe the dynamics of these interactions and uncover mechanisms underlying immune effector cell infiltration, cytotoxic activity, exhaustion, and persistence in concert with the dynamic responses of tumor and microenvironmental phenotypes.
Identifying novel immune effector cell targets in gliomas using single cell sequencing
Our laboratory leverages single cell transcriptomics to understand the gene regulatory programs that drive the expression of cell surface targets in molecularly-defined gliomas. In contrast to traditional approaches, this strategy enables transcriptome-wide evaluation of target profiles across heterogeneous tumor cell populations and the background brain environment. From these data, we are building comprehensive atlases of the targeting landscape in these diseases to inform the design of next-generation combinatorial immune effector cell targeting strategies that will enhance our ability to overcome tumor heterogeneity. Using diverse model systems, including patient-derived glioma organoids, cell lines, and mice, we then probe the dynamics of these transcriptional programs under therapeutic pressure with panels of novel immune effector cell constructs. Using these systems, we have uncovered convergent transcriptional metaprograms of glioma cell responses to immune effector cell therapies, and we are now exploring targeting strategies to translate these opportunities into novel therapies.
Deciphering microenvironmental mediators of immune effector cell function and therapeutic resistance with spatial multiomics
In patients with brain tumors, how does an infused cellular therapy reach its target tumor cell population in the central nervous system? Despite the fundamental importance of this question, we have a limited understanding of the physical pathways and molecular mechanisms by which engineered T cell therapies infiltrate brain tumors. Evolving spatial multiomics platforms now offer the ability to interrogate these questions with unprecedented resolution and depth. In collaboration with colleagues in neurosurgery, neuro-oncology, and neuropathology, our laboratory conducts spatial multiomic profiling of patient tumor samples treated in immune effector cell clinical trials to unravel the molecular architecture of these interactions in situ. In model systems, we expand on these observations in collaboration with longstanding bioinformatics collaborators to understand the influence of spatial architecture and cellular neighborhoods on transcriptional dynamics and immune effector cell function in situ.
Select Publications
Greenwald AC, Darnell NG, Hoefflin R, Simkin D, Mount CW, Gonzalez Castro LN, Harnik Y, Dumont S, Hirsch D, Nomura M, Talpir T, Kedmi M, Goliand I, Medici G, Laffy J, Li B, Mangena V, Keren-Shaul H, Weller M, Addadi Y, Neidert MC, Suvà ML, Tirosh I. Integrative spatial analysis reveals a multi-layered organization of glioblastoma. Cell. 2024 May 9;187(10):2485-2501.e26.
Choi BD, Gerstner ER, Frigault MJ, Leick MB, Mount CW, Balaj L, Nikiforow S, Carter BS, Curry WT, Gallagher K, Maus MV. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N Engl J Med. 2024 Apr 11;390(14):1290-1298.
Theruvath J, Sotillo E, Mount CW, Graef CM, Delaidelli A, Heitzeneder S, Labanieh L, Dhingra S, Leruste A, Majzner RG, Xu P, Mueller S, Yecies DW, Finetti MA, Williamson D, Johann PD, Kool M, Pfister S, Hasselblatt M, Frühwald MC, Delattre O, Surdez D, Bourdeaut F, Puget S, Zaidi S, Mitra SS, Cheshier S, Sorensen PH, Monje M, Mackall CL. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020 May;26(5):712-719.
Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, Labanieh L, Hulleman E, Woo PJ, Rietberg SP, Vogel H, Monje M, Mackall CL. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018 May;24(5):572-579.
Research Image
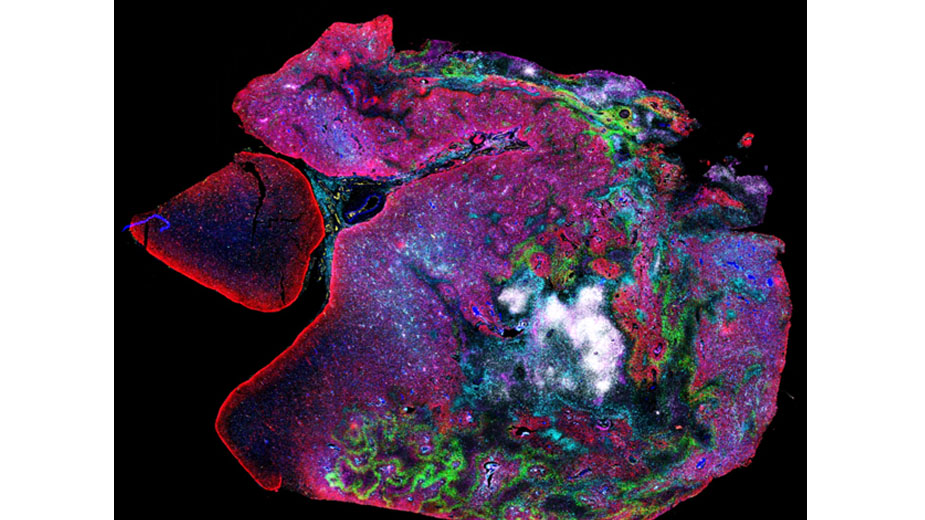
Highly multiplexed spatial proteomic profiling (CODEX) of a CAR-T cell treated patient tumor specimen.
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
Support the Krantz Family Center for Cancer Research
When you support us you are enabling discoveries that will lead to effective new weapons in the battle against cancer.
