Krantz Family Center for Cancer Research
Shi Laboratory
Contact Information
Shi Laboratory
(opens fall 2025)
Diana D. Shi, MD
Faculty Member
Mass General Brigham Cancer Institute
Harvard Medical School
Program Affiliations
Krantz Family Center for Cancer Research
Explore the Shi Lab
Overview

Gliomas are the most common primary brain tumors, a subset of which have mutations in the gene isocitrate dehydrogenase 1 (IDH1). Though IDH-mutant gliomas are distinct in their clinical behavior, much remains unknown about how IDH mutations alter glioma biology. The Shi laboratory aims to understand how IDH mutations induce metabolic, epigenetic, and transcriptional changes in glioma in order to develop new treatments for this disease. To achieve this goal, we have developed and use genetically engineered mouse models that capture key biology of IDH mutations in glioma. In addition, we employ engineered patient-derived cell and animal models to test preclinical treatment strategies that are poised for clinical translation. We couple these studies with functional genomic screening, pharmacologic screening, and mechanistic experimental cell biology to identify new therapies for both IDH-mutant gliomas and other epigenetically and/or metabolically dysregulated cancers.
Research Summary
Gliomas are the most common primary brain tumors in adults, a subset of which have mutations in the metabolic gene isocitrate dehydrogenase 1 (IDH1). Cancer-associated IDH1 mutants are gain-of-function mutations that produce the oncometabolite 2-hydroxyglutarate [(R)-2HG]. (R)-2HG is thought to transform cells by competitively inhibiting 2OG-dependent dioxygenases, many of which are epigenetic regulators, given the structural similarity of (R)-2HG and 2OG. However, much remains unknown about how these downstream effects of mIDH drive tumor growth and therapeutic efficacy. My research interests center on understanding the biology of IDH-mutant gliomas and their response to treatment, and using that information to uncover central functions of epigenetic and metabolic reprogramming in cancer. To address these questions, we made a genetically engineered mouse (GEM) model that can be used to study key IDH-mutant biology in glioma. We have leveraged this GEM and other models to show that IDH-mutant gliomas are sensitive to de novo pyrimidine synthesis inhibitors (e.g. dihydroorotate dehydrogenase (DHODH) inhibitors) due to an increased susceptibility of IDH-mutant cells to replication stress caused by these drugs. Ongoing work aims to build on these findings across three areas of investigation:
Identifying and functionally assessing mutant IDH inhibitor response and resistance in glioma
The mutant IDH (mIDH) inhibitor vorasidenib has been recently FDA approved for the treatment of select IDH-mutant glioma patients and has become standard-of-care. However, response to mIDH inhibitors is heterogeneous, and our understanding of how these drugs work in glioma has been severely limited by a lack of faithful animal models that respond to mIDH inhibition. To address this gap, we developed an IDH-mutant GEM model and found that it responds to mIDH inhibitor treatment. We are now using this model to perform single-cell multiomics sequencing, spatial transcriptomics, metabolomics, and methylation profiling to understand mechanisms underlying response to mIDH inhibitors in glioma. Our goal is to leverage the knowledge gained from these studies to perform functional testing to understand how IDH-mutant gliomas respond, or become resistant to, mIDH inhibitor therapy.
Understanding the interaction between mutant IDH inhibition and radiation
While many patients benefit from mIDH inhibitor therapy, patients inevitably progress and require second-line treatment with chemotherapy and/or radiation. While mIDH is known to cause altered response to DNA damage, it is unknown how mIDH inhibition affects efficacy of chemoradiation. We are using our IDH-mutant GEM to perform efficacy and molecular profiling studies to understand how mIDH inhibitors alter chemoradiation efficacy. We are profiling both tumor-intrinsic and immune microenvironmental effects of mIDH (and mIDH inhibition) that affect response to radiation and alkylating agents. Results from this work may inform rational concurrent and/or sequential treatment regimens combining mIDH inhibitors, radiation, and chemotherapy.
Identifying how epigenetic alterations affect response to therapeutic stress
IDH-mutant glioma patients who are resistant to mIDH inhibition require effective alternative treatment options. Our previous work identified that DHODH inhibitors are effective in IDH-mutant gliomas due to a sensitivity to replication stress conferred by mIDH. We have since identified that inhibition of specific dioxygenases mediates this sensitivity to replication stress, and we are interested in (1) whether inhibition of these dioxygenases sensitizes to drugs that induce replication stress across non-glioma cancer types, and (2) how epigenetic alterations functionally alter replication stress response.
Taken together, these areas of investigation aim to pursue mechanistic investigations with faithful preclinical models to inform rational therapeutic strategies for IDH-mutant glioma patients. More broadly, our goal is to use insights from IDH-mutant glioma to understand how metabolic and epigenetic alterations affect tumor biology and therapeutic stress across cancer types.
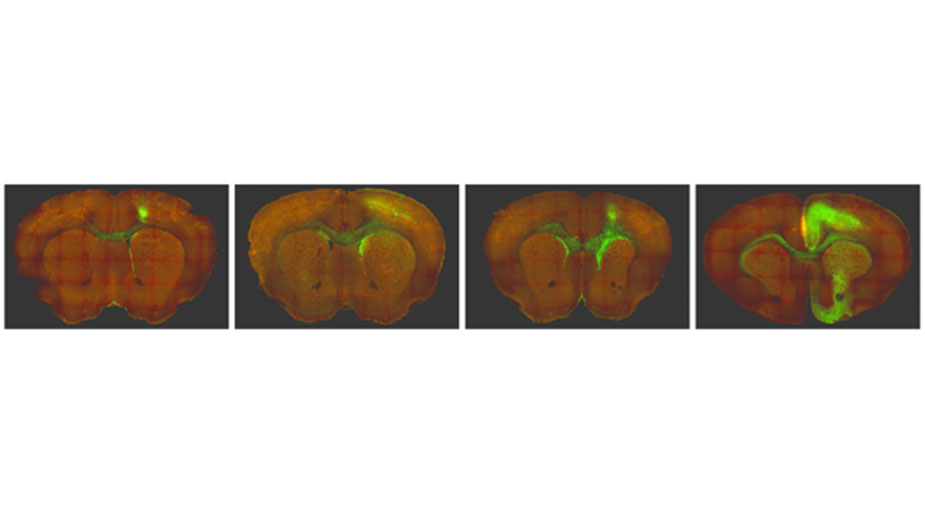
Research Image
Green fluorescent protein (GFP) imaging of glioma tumor development over time in a genetically engineered mouse model of IDH-mutant glioma developed by the lab. Glioma tumor cells are GFP+.
Select Publications
Savani MR, El Shami M, Gattie LC, Smith BC, Hicks WH, Oken SS, Zacharias LG, Martin-Sandoval MS, Montgomery EY, Xiao Y, Shi DD, Rich JN, Richardson TE, Zinn PO, Lega BC, Mathews TP, DeBerardinis RJ, McBrayer SK, Abdullah KG. Stable isotope tracing in human plasma-like medium reveals metabolic and immune modulation of the glioblastoma microenvironment. bioRxiv [Preprint]. 2025 Apr 26:2023.05.29.542774.
Sternisha AC, Li H, Traylor JI, Guo L, Jun JH, Zhao X, Gajendra K, Ouyang Q, Schmidt M, Fleishman M, Shi DD, Savani MR, Xiao Y, Lee JH, Zacharias LG, Mathews TP, Gordillo R, Kim YJ, Xu L, Doench JG, Koduri V, Abdullah KG, Banaszynski LA, Agathocleous M, DeBerardinis RJ, Morrow EM, McBrayer SK. A transcriptional biosensor reveals mechanisms of ⍺-ketoglutarate signaling to chromatin. BioRxiv. [Preprint]. 2025 April 21.
Wu MJ, Kondo H, Kammula AV, Shi L, Xiao Y, Dhiab S, Xu Q, Slater CJ, Avila OI, Merritt J, Kato H, Kattel P, Sussman J, Gritti I, Eccleston J, Sun Y, Cho HM, Olander K, Katsuda T, Shi DD, Savani MR, Smith BC, Cleary JM, Mostoslavsky R, Vijay V, Kitagawa Y, Wakimoto H, Jenkins RW, Yates KB, Paik J, Tassinari A, Saatcioglu DH, Tron AE, Haas W, Cahill D, McBrayer SK, Manguso RT, Bardeesy N. Mutant IDH1 inhibition induces dsDNA sensing to activate tumor immunity. Science. 2024 Jul 12;385(6705):eadl6173.
Shi DD, Lee JH, Wang AC, Khanal J, Gao W, Kaelin WG Jr, McBrayer SK. Protocol to establish a genetically engineered mouse model of IDH1-mutant astrocytoma. STAR Protoc. 2023 May 6;4(2):102281.
Shi DD, Savani MR, Levitt MM, Wang AC, Endress JE, Bird CE, Buehler J, Stopka SA, Regan MS, Lin YF, Puliyappadamba VT, Gao W, Khanal J, Evans L, Lee JH, Guo L, Xiao Y, Xu M, Huang B, Jennings RB, Bonal DM, Martin-Sandoval MS, Dang T, Gattie LC, Cameron AB, Lee S, Asara JM, Kornblum HI, Mak TW, Looper RE, Nguyen QD, Signoretti S, Gradl S, Sutter A, Jeffers M, Janzer A, Lehrman MA, Zacharias LG, Mathews TP, Losman JA, Richardson TE, Cahill DP, DeBerardinis RJ, Ligon KL, Xu L, Ly P, Agar NYR, Abdullah KG, Harris IS, Kaelin WG Jr, McBrayer SK. De novo pyrimidine synthesis is a targetable vulnerability in IDH mutant glioma. Cancer Cell. 2022 Sep 12;40(9):939-956.e16.
Shi DD, Youssef GC, Nassar AH, Lim-Fat MJ, Ligon KL, Wen PY, Rahman R. Improved survival among females and association with lymphopenia in patients with newly diagnosed glioblastoma. Neuro Oncol. 2022 Nov 2;24(11):2005-2007.
Lab Members
Alexander Tsai, BA
Dorothy Junginger, BA
Diana D. Shi, MD
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
Support the Krantz Family Center for Cancer Research
When you support us you are enabling discoveries that will lead to effective new weapons in the battle against cancer.
