Research Spotlight: Comparison of Single-Cell Profiling Methods Reveals Strengths and Limitations
The study will help researchers more efficiently approach projects employing single cell RNA sequencing on human cancer biopsies.
Department of Medicine
Contact Information
CNY 149-8
149 13th Street
Charlestown,
MA
02129
Phone: 617-726-5663
Fax: 617-726-5671
Email: aarnaout1@mgh.harvard.edu
Hours: Monday–Friday, 8:00 am–6:00 pm
Grants Manager: Diane DeAngelis
Phone: 617-724-1551
Email: ddeangelis2@mgh.harvard.edu
Laboratory Manager: Zhiping Ding
Phone: 617-724-9875
Email: zding@partners.org
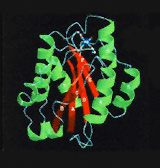
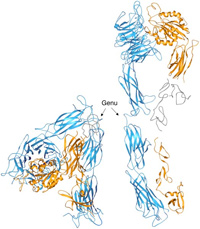
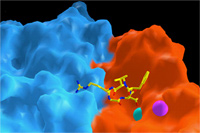
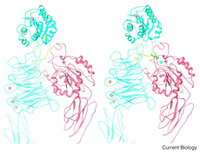
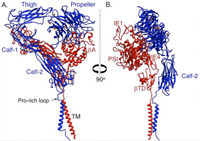
A major research goal of the Arnaout Laboratory is to elucidate the structure and function of integrins, cell adhesion receptors that play vital roles in normal physiology and disease and use the derived information in structure-based design of new and safer anti-integrin drugs targeting heart disease, fibrosis, and cancer.
Other research interests include elucidating mechanisms underlying cyst formation in Autosomal Dominant Polycystic Kidney Disease, transcriptional regulation of hematopoiesis, mechanisms of kidney regeneration, and design of microfluidic dialysis devices.
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
If you are interested in applying for a postdoctoral position, or are a Harvard PhD student interested in a laboratory rotation, please e-mail your CV (for student and postdoctoral) and reference letters (for postdoctoral) to: aarnaout1@mgh.harvard.edu
See a list of publications from this investigator.
Every day, our clinicians and scientists chart new terrain in biomedical research to treat and prevent human disease and advance patient care.
Your support of Nephrology helps us provide the best possible care today and expand what will be possible tomorrow.
We offer innovative, high-quality medical care, trains future medical leaders, and produces research that advances science and improves care.
The study will help researchers more efficiently approach projects employing single cell RNA sequencing on human cancer biopsies.
Researchers used computer modeling to evaluate the potential clinical impact and cost-effectiveness of administering long-acting, injectable antibodies to infants from birth to prevent HIV infection during breastfeeding.
Given uncertainties about future PEPFAR funding, researchers modeled the impact of abrupt PEPFAR cutbacks in South Africa
Write a short description of the Research Spotlight.
Uncontrolled blood pressure puts people at increased risk of developing heart disease, brain disease, and kidney disease, yet only one in four people have their blood pressure under good control.
Shadmehr (Shawn) Demehri, MD, PhD, is the corresponding author of a paper published in Cancer Cell, “Commensal papillomavirus immunity preserves the homeostasis of highly mutated normal skin.”
The Division of Nephrology at Massachusetts General Hospital is a leading provider of services for patients with kidney disease, including diagnosis and management of kidney diseases and medical management of renal transplantation.