Krantz Family Center for Cancer Research
Bod Lab


Contact Information
Bod Laboratory
Lloyd Bod, PhD
Assistant Professor of Medicine
Mass General Brigham Cancer Institute
Harvard Medical School
Program Affiliations
Krantz Family Center for Cancer Research
Explore the Bod Lab
2024 Krantz Awards Recipient
2024 Quantum Award: Boosting immunotherapy response while eradicating toxicity
Team: Lloyd Bod, PhD, William L. Hwang, MD, PhD, David T. Ting, MD, and Alexandra-Chloe Villani, PhD.
Learn about the team's project and the Krantz Awards
Research Summary
Immunotherapies have demonstrated remarkable clinical success in the treatment of various cancers mainly by boosting the function of endogenous T cells to attack neoplastic cells. Unfortunately, the frequency of patients responding to these therapies is modest and a significant fraction of patients develop severe immune-related adverse events. These observations have catalyzed a more thorough investigation of other cell types in the tumor microenvironment that could be targeted to increase treatment efficacy while mitigating toxicity. B cells are an important arm of the adaptive immune system frequently infiltrating solid tumors, however, their function on cancer progression has not been sufficiently explored.
The Bod laboratory focuses on deciphering the landscape of phenotypic and functional B cell states within tumors. In particular, we are interested in exploring which B cell subset is favorable or detrimental for cancer progression, and by which mechanisms these B cells control tumor growth. Our thorough examination of the B cell response towards cancer aims to provide a new angle to harness the anti-tumor immune response more effectively.
Research Projects
Historically, B cells have been at the forefront of research in allergies, infectious diseases, and vaccines. Beyond mediating the humoral response, B cells are potent antigen presenting cells (APCs). They can provide co-stimulatory or co- inhibitory signals and secrete cytokines and chemokines that regulate functions of other cell types including effector T cells.
However, the role of B cells in the cancer scenario is unclear. While some studies have shown that B cells are critical for promoting anti-tumor immunity, others report that they may play a detrimental role, favoring relapse and metastasis. Indeed, on one hand, B cells form tertiary lymphoid structures (TLS) in the context of successful immune checkpoint blockade (ICB) therapy in human cancer patients, suggesting that B cells and TLS provide critical help to promote antitumor immunity and inhibit tumor growth.
On the other hand, B cells may also play an inhibitory role through the expression of soluble and/or inhibitory molecules on their surface which contribute to dismantle the anti-tumor T cell immunity. Whether the paradoxical effects of B cells in these settings is due to their functional diversity or distinct roles within different tumor types remains to be elucidated.
A more comprehensive understanding of B cell heterogeneity in tumors will allow us to identify B cell subsets and their respective functionality arising during different stages of tumor growth and regulating anti-tumor immunity. Growing evidence suggests that lymphocytes occupy a vast and continuous landscape of possible cellular states, as opposed to the idea of disconnected discrete subtypes. Recent advances in genomic analysis and sophisticated computational methods are enabling us to explore such diversity and are transforming our comprehension of immunology. Using such approaches, the lab aims to generate new insights into the role of B cells in inducing and regulating anti-tumor immunity. The main axes of research in our laboratory are:
The main axes of research in our laboratory are:
- Deciphering the landscape of B cell states within the tumor microenvironment using multi-omics technologies. Our goal is to establish an atlas of B cell states in cancer, and to thoroughly interpret the spatial, transcriptomic, and epigenetic status of B cells in different contexts (e.g., different tumor types, healthy tissues, post-treatment with immune checkpoint blockade therapy, chemotherapy, or radiotherapy).
- Identifying B cell-specific biomarkers and/or -targets in cancer. Using genetic and genomics approaches, we aim to explore potential B cell biomarkers and novel targets that are expressed on B cells, which may synergize with T cellbased checkpoint blockade therapy to enhance anti-tumor immunity.
- Dissecting the underlying cellular and molecular mechanisms that govern the B cell response to cancer. The tumor microenvironment is layered with multiple tissular, cellular and molecular components which are associated with distinct tumor-promoting or -inhibiting mechanisms, and ultimately, open distinct therapeutic windows. We are interested in elucidating how B cells integrate these components and how the anti-tumor B cell response evolves in response to these signals.
Publications
Selected Publications
Bod L, Kye YC, Shi J, Torlai Triglia E, Schnell A, Fessler J, Kuchroo JR, Barilla RM, Zaghouani S, Christian E, Delorey TM, Mohib K, Xiao S,Rothstein DM, Rozenblatt-Rosen O, Sharpe AH, Apetoh L, Regev A, Kuchroo V.K. B-cell specific checkpoint molecules that regulate anti-tumor immunity. In Press at Nature 2023.
Schnell A, Bod L, Madi A, Kuchroo VK. (2020). The yin and yang of co-inhibitory receptors: toward antitumor immunity without autoimmunity. Cell Res, 30(4), 285-299.
Xiao S, Bod L, Pochet N, Kota SB, Hu, D, Madi A, Kilpatrick J, Shi J, Ho A, Zhang H, Sobel R, Weiner HL, Strom TB, Quintana FJ, Joller N, Kuchroo VK. (2020). Checkpoint Receptor TIGIT Expressed on Tim-1(+) B Cells Regulates Tissue Inflammation. Cell Rep, 32(2), 107892.
Bod L, Douguet L, Auffray C, Lengagne R, Bekkat F, Rondeau E, Molinier-Frenkel V, Castellano F, Richard Y, Prevost-Blondel A. (2018). IL-4-Induced Gene 1: A Negative Immune Checkpoint Controlling B Cell Differentiation and Activation. J Immunol, 200(3), 1027-1038.
Bod L, Lengagne R, Wrobel L, Ramspott JP, Kato M, Avril MF, Castellano F, Molinier-Frenkel V, Prevost-Blondel A. (2017). IL4- induced gene 1 promotes tumor growth by shaping the immune microenvironment in melanoma. Oncoimmunology, 6(3), e1278331.
Research Image
While existing anti-cancer immunotherapies mainly engage effector T cells, harnessing both arms of the adaptive immune system might be more favorable. Illustrated by the mosaic of diverse B cell states, B cells are a highly dynamic cell population in the tumor microenvironment (TME) favoring or impeding tumor growth.
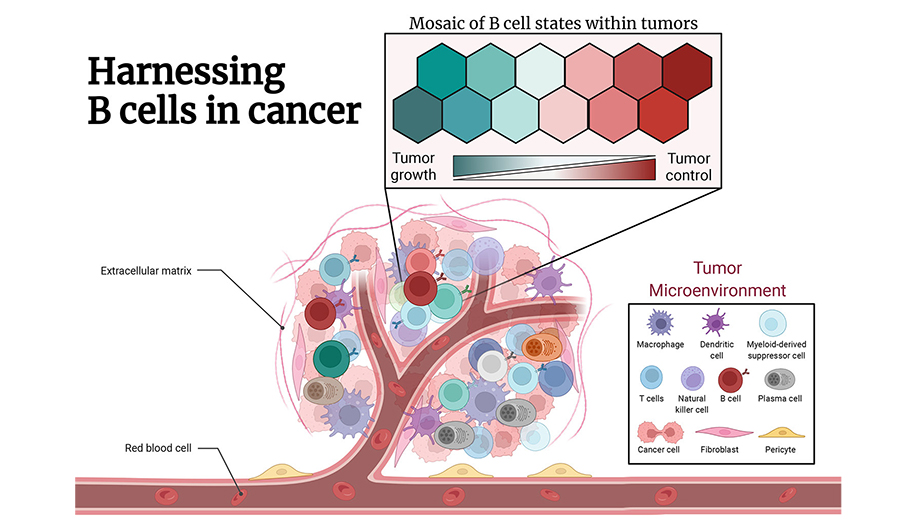
In our lab, we want to thoroughly dissect the diverse and complex functions of TME-associated B cells to pave the way for new therapeutic avenues and improve the anti-cancer immune response. Adapted from “Tumor Microenvironment”, by BioRender.com (2022).
Our Researchers
Lloyd Bod, PhD
Principal Investigator- Vanessa Daniels, BSc
- Alice Dejoux, PhD
- Kaiyao Li, MSc
- Kassiana Mafra, PhD
- Ranjan Maskey, BSc
- Anna Moustaid*
- Chambit Paik, BSc
- Aditya Patil, MSc*
- Fenisha Shah, MSc
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
Support the Krantz Family Center for Cancer Research
When you support us you are enabling discoveries that will lead to effective new weapons in the battle against cancer.
