Krantz Family Center for Cancer Research
Rheinbay Lab


Contact Information
Esther Rheinbay, PhD
Assistant Professor of Medicine
Mass General Brigham Cancer Institute
Harvard Medical School
Program Affiliations
Krantz Family Center for Cancer Research
Explore the Rheinbay Lab
2024 Krantz Awards Recipient
2024 Breakthrough Award: Targeting intrinsically disordered protein domains in cancer
Team: Esther Rheinbay, PhD and Miguel N. Rivera, MD.
Learn about the team's project and the Krantz Awards
Research Summary
Most known genomic drivers of cancer are in coding genes, affecting the encoded protein’s interaction with other proteins, DNA or biological compounds. Recent advances in DNA sequencing technology have made it possible to study non-coding regions that regulate these protein-coding genes. Several cancer drivers have been identified and characterized in these regulatory regions, however, this genomic territory remains relatively unexplored in human tumors. The Rheinbay laboratory concentrates on identifying and functionally characterizing these non-coding drivers in the sequences of tumor whole genomes through development of novel analysis strategies and collaborations with experimental investigators.
We are also interested in the contribution of the sex chromosomes, especially the Y chromosome, to cancer. Loss of Y is known to be associated with morbidity and mortality in aging men, yet its role in tumors is largely unclear. Much of this is due to technical challenges that our group aims to solve. Understanding the driver genes on the sex chromosomes will help us explain differences in male and female tumors, and forge a path to more effective, sex-informed treatment.
Research Projects
Regulatory driver mutations in cancer genomes
Genomic cancer driver discovery has traditionally focused on protein-coding genes (the human exome), and large-scale sequencing of these genes in thousands of tumors has led to the discovery of novel frequently altered genes. However, exome sequencing focused only on coding genes does not allow analysis of non-coding regions in the human genome. Proteincoding genes are regulated by several types of genomic elements that control their expression (promoters, distal enhancers and boundary elements), translation (5’UTRs) and mRNA stability (3’UTRs). Alterations in the DNA sequence of these elements thus directly affect the expression and regulation of the target gene. Several such non-coding elements have been identified as recurrently altered in human cancer, and functionally characterized, although these non-coding drivers appear infrequent compared to protein-coding oncogenes and tumor suppressors. One reason might be that gene regulation is highly tissue-specific, and therefore driver alterations in non-coding regions might create a fitness advantage in only a single tumor type. Finding such a specific driver requires a sufficient number of whole genomes from this tumor type. With recent advances in DNA sequencing technology and an increasing number of whole cancer genomes available for analysis, we are just starting to map out and characterize regulatory driver alterations. The Rheinbay laboratory works on the development of novel methods to identify non-coding driver candidates using genomic and epigenomic sources of information, and to understand their impact on tumor initiation, progression and treatment resistance through collaborations with experimental colleagues.
Role of the sex chromosomes in cancer
Cancer affects men and women disparately, with strong differences in incidence and outcome in some tumor types. Human sex is determined by the sex chromosomes X and Y. Because men only have one X chromosome, they are particularly vulnerable to congenital and acquired somatic variants in X-linked genes. It has been shown that both sex chromosomes can be lost in both normal blood cells with age, as well as certain tumor cells. Yet the meaning of Y chromosome loss, and possible cancer genes on this chromosome, are poorly understood. This is because Y is technically challenging to study with commonly used ‘omics’ profiling approaches. We develop analysis strategies and methods to tackle these technical challenges and use them to find new X and Y-linked drivers in published tumor genome sequences. Our goal is to identify sex-specific, and potentially targetable, vulnerabilities in human cancer.
Publications
Selected Publications
Qi M, Pang J, Mitsiades I, Lane AA, Rheinbay E. Loss of chromosome Y in primary tumors. Cell, 2023; 186(14): 3125-3136.
Qi M, Nayar U, Ludwig, LS, Wagle N, Rheinbay E. cDNA-detector: detection and removal of cDNA contamination in DNA sequencing libraries. BMC Bioinformatics. 2021. 22:611
Rheinbay, E.*, Nielsen, M.M.*, Abascal, F.* et al. Analyses of non-coding somatic drivers in 2,658 cancer whole genomes. Nature 578, 102–111 (2020).
Rheinbay E, Parasuraman P, Grimsby J, et al. Recurrent and functional regulatory mutations in breast cancer. Nature. 2017;547:55-60.
Suva ML*, Rheinbay E*, Gillespie SM, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580-94.
*Equal contribution
We're Hiring! Postdoctoral Position
Postdoctoral Position in the Rheinbay Laboratory
Open position in computational cancer genomics to study cancer drivers and gender differences in cancer.
We are looking for a self-motivated postdoctoral researcher with a strong background in computational science and experience with large data sets. The successful candidate will join an interdisciplinary team working on rigorous analysis of next generation sequencing data (DNA, RNA, chromatin) from tumor samples, and development of analysis tools that will be shared with the research community. This is a unique training opportunity with access to resources at the Mass General Brigham Cancer Institute, Harvard Medical School and the Broad Institute.
Qualifications:
- PhD in computational biology, bioinformatics, computer science, physics or applied math
- Strong publication record, communication and writing skills, fluency in English
- Excellent programming skills in Python and R, experience with cloud computing environments preferred
- Ability to work together with multi-disciplinary teams consisting of physicians, experimental scientists, statisticians and software engineers
- Experience with NGS data processing and analysis is desired
Interested candidates should submit a cover letter, curriculum vitae, research background and interests and contact information for three references to: Dr. Esther Rheinbay, erheinbay@mgh.harvard.edu.
We're Hiring! Data Analyst Position
Data Analyst - Cancer Computational Biology
Emails:
erheinbay@mgh.harvard.edu
mslawrence@mgh.harvard.edu
Unique opportunity to join an interdisciplinary team bridging the Harvard Medical School, the Massachusetts General Hospital, and the Broad Institute of Harvard and MIT. The Rheinbay Lab and Lawrence Lab at the Mass General Brigham Cancer Institute seek well-qualified candidates to join a team of computational biologists working at the forefront of cancer research and treatment. We use computation as a powerful microscope to study both the fundamental biology of cancer initiation and progression, as well the diagnosis and treatment of cancer patients in the hospital setting.
Current research interests:
- Cancer driver genes: tumors grow because of specific driver mutations that deactivate tumor suppressors or activate oncogenes. We are working to complete our understanding of the full catalog of cancer's "box of tricks".
- Resistance to targeted therapies: single drugs targeting specific driver mutations can be effective for a while, but the cancer invariably discovers a work-around. We are actively investigating mechanisms of drug resistance and how to combat it.
- Single-cell sequencing: New approaches allow us to dissect a tumor down to single cells and investigate the RNA expression or DNA mutations in each cell. Understanding intratumoral heterogeneity is shedding new light on cancer progression and patient outcomes.
- Liquid biopsies: novel state-of-the-art technologies are starting to allow us to monitor the progression of cancer (both before, during, and after treatment) through a simple blood draw. We are actively working to overcome analytical challenges inherent in the study of circulating tumor cells (CTCs) and cell-free circulating tumor DNA (ctDNA).
- Mutational processes: our genomes accumulate mutations from environmental agents such as ultraviolet radiation and tobacco smoke, as well as from intrinsic processes like errors during DNA replication. Studying these mutational background patterns can tell us what repair pathways are broken in a specific tumor, perhaps pointing the way to an effective genotoxic therapy. We are working to develop novel DNA sequencing technologies for studying mutagenesis in model systems
- Genomics of sex chromosomes in cancer: understand the genetic underpinnings of different incidence and outcome between men and women patients
Principal Duties:
- Work with Cancer Institute researchers to understand experimental procedures and the kinds of data produced (e.g. DNA sequencing, RNA sequencing, epigenetic readouts, clinical outcome annotations). Meet and discuss with clinical and experimental colleagues to identify analytical challenges and goals.
- Apply existing and novel algorithms to cancer data sets, analyze data quality, critically review and analyze results, communicate results to biologists, computational biologists, software engineers and clinicians.
- Explore novel data visualization tools, with emphasis on integrating diverse data types and extracting clinically relevant insights.
- Contribute to scientific writing and creation of data figures to be included in research publications reporting novel discoveries made in the lab and clinic.
Required skills:
- B.A/B.S. in one of Computational Biology, Bioinformatics, Biology, Computer Science, Mathematics, Physics, or a related quantitative discipline.
- Independent, self-motivated drive to push research forward.
- Excellent programming skills (using any of Matlab, R, Java, Python, Perl, C, etc.)
- Nimble approach to programming and data analysis, with an emphasis on simple, intuitive, reasoning: quickly open unfamiliar datasets, generate simple visualizations to project the data onto our brains as usefully as possible, to stimulate hypothesis generation and the next steps of the analysis.
- Comfort using Word, Excel, Powerpoint or Google Suite tools to communicate results between team members.
- Ability to work together with multi-disciplinary teams comprising physicians, biologists, statisticians, and software engineers.
- Strong organizational and record-keeping skills
- Fluency in spoken and written English
- Experience in "machine learning" welcomed
Research Image
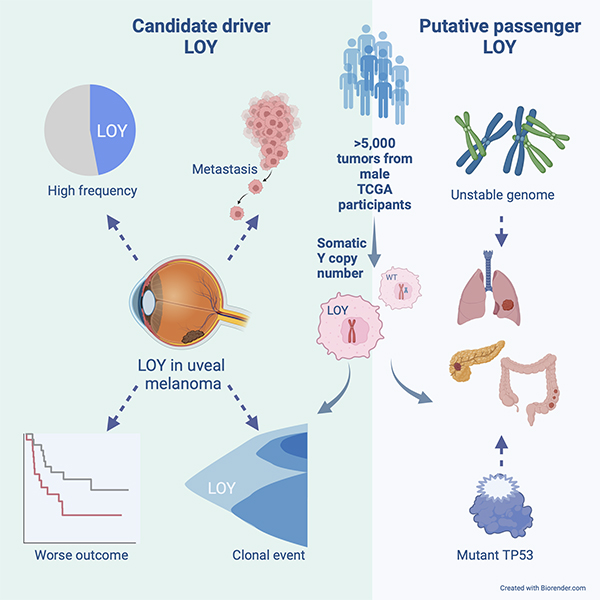
Y chromosome loss in cancer can be a driver (left) or passenger (right) event.
Our Researchers
Esther Rheinbay, PhD
Principal InvestigatorGroup Members
- Luis Antonio Corchete Sánchez, PhD*
- Preshita Dave**
- Philipp Hähnel, PhD***
- Ema Klefti
- Garrett Lam†
- Andrey Leshchiner
- Xavi Loinaz††
- Irene Rin Mitsiades
- Andres Fillizzola Ortiz
- Gengchao Wang, PhD
* Co-mentored with Langenau lab
** Co-mentored with Lawrence lab
*** Co-mentored with Brastianos Lab
† Co-mentored with Ellisen Lab
††Co-mentored with Getz Lab
Krantz Family Center for Cancer Research
The scientific engine for discovery for the Mass General Brigham Cancer Institute.
Support the Krantz Family Center for Cancer Research
When you support us you are enabling discoveries that will lead to effective new weapons in the battle against cancer.
