Metabolism Unit: Research Studies
What We Study
Metabolism Unit faculty have advanced our understanding of the nutritional regulation of hypothalamic function, nutrient trafficking and substrate metabolism and the mechanisms/consequences of ectopic adipose tissue accumulation in obesity and acquired lipodystrophies. Additionally, Metabolism Unit faculty have advanced knowledge on the interplay between innate immune activation, adipose tissue dysfunction and cardiometabolic disease in special populations, including people with obesity, metabolic syndrome and HIV. Based on this work, the unit is exploring novel hormonal and immune strategies to prevent or treat metabolic and cardiovascular disease in representative patient populations.
How Your Participation in Research Matters
Your participation in Metabolism Unit studies matters because:
- Research can help provide insights into your own metabolic health and contribute to the body of knowledge scientists and clinicians use to help you and others navigate complex health issues
- Your participation can help scientists learn more about metabolic health issues and discover future therapies and treatment strategies to help others
Current Research Studies
The Metabolism Unit is currently conducting a number of clinical research studies. You can learn more about each study by selecting the titles below.
Elucidating Growth Hormone Dynamics at Stages of Progressive Nonalcoholic Fatty Liver Disease
Principal Investigators: Takara Stanley, MD, and Steven Grinspoon, MD
Co-Investigators: Allison Arpante, NP
Funding: Theratechnologies, Inc.
Contact Information: Michele Young, mcyoung@mgh.harvard.edu
ClinicalTrials.gov: NCT06166797
 The purpose of this study is to investigate relationships between the body's growth hormone "axis" and metabolism-dysregulation associated steatotic liver disease (MASLD, previously called nonalcoholic fatty liver disease [NAFLD]). The growth hormone "axis" includes the hormones growth hormone and insulin-like growth factor 1, and associated proteins. We hypothesize that there will be a relationship such that people with more advanced MASLD will have greater impairments in the growth hormone axis. There are no treatments associated with this research study.
The purpose of this study is to investigate relationships between the body's growth hormone "axis" and metabolism-dysregulation associated steatotic liver disease (MASLD, previously called nonalcoholic fatty liver disease [NAFLD]). The growth hormone "axis" includes the hormones growth hormone and insulin-like growth factor 1, and associated proteins. We hypothesize that there will be a relationship such that people with more advanced MASLD will have greater impairments in the growth hormone axis. There are no treatments associated with this research study.
Interested in participating in this research? Learn more here.
A 12-Month Randomized, Multicenter, Double-Blind, Placebo-Controlled Phase 3 Study to Evaluate the Safety and Efficacy of Daily Subcutaneous Metreleptin Treatment in Subjects with Partial Lipodystrophy
Principal Investigators: Lindsay Fourman, MD
Funding: Industry Funded – Amryt Pharma
Contact Information: lfourman@partners.org
ClinicalTrials.gov: NCT05164341
Lipodystrophy (LD) is a rare, heterogeneous group of syndromes characterized by a complete or partial loss or absence of subcutaneous adipose tissue (fat). Because of the loss of adipose tissue, levels of the adipocyte-secreted hormone leptin in patients with LD are very low. This disrupts the body’s system for regulating energy use and storage. Metabolic abnormalities often occur with LD, including insulin resistance with resultant hyperinsulinemia and diabetes; hepatic steatosis or steatohepatitis; and dyslipidemia with severe hypertriglyceridemia. This study focuses on patients with familial partial LD (FPLD). Individuals with FPLD often have reduced subcutaneous fat in the arms and legs, while the trunk regions may or may not have loss of fat. FPLD may run in families or be attributed to a specific genetic mutation. To help people with FPLD, we are exploring treating FPLD with metreleptin, which is a recombinant human leptin analog that is indicated as a replacement therapy to treat the complications of leptin deficiency. Metreleptin has been approved by the Food and Drug Administration (FDA) for treating patients with generalized LD who have complete loss of fat. While it is approved in the EU for both patients with generalized LD and FPLD, the use of metreleptin in patients with FPLD has not yet been approved by the FDA. This study will be conducted as a randomized, double-blind, placebo-controlled study and will provide useful efficacy and safety information for the use of metreleptin in patients with FPLD.
If you are interested in participating in this research study, please contact srusso4@mgh.harvard.edu.
MIneralocorticoid Receptor Antagonism for CardiovascuLar hEalth in HIV (The MIRACLE HIV Study)
Principal Investigators: Steven Grinspoon, MD; Gail Adler, MD, PhD
Co-Investigators: Suman Srinivasa, MD; Allie Walpert, FNP
Funding: NIH R01 DK049302
Contact Information: SGRINSPOON@mgh.harvard.edu; SSRINIVASA@mgh.harvard.edu; awalpert@mgh.harvard.edu
ClinicalTrials.gov: NCT02740179
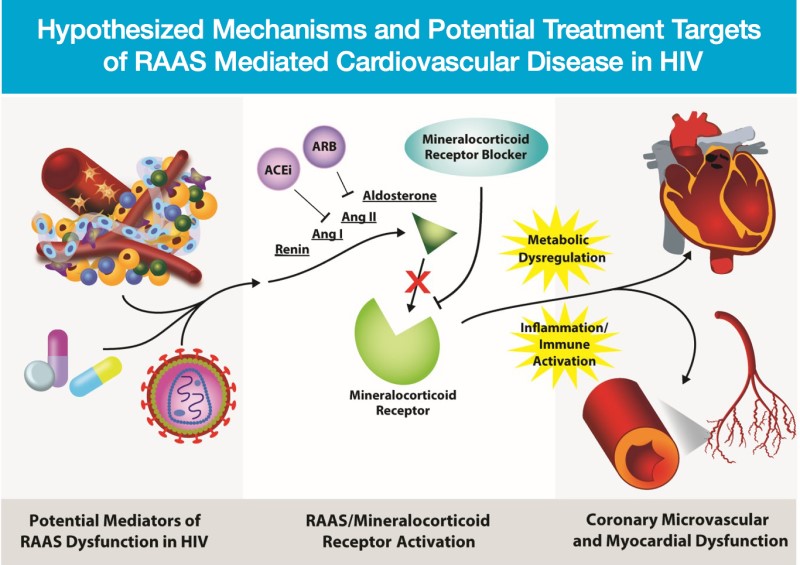
People with HIV treated with antiretroviral medications are living longer, but have an increased risk of heart disease when compared to people without HIV. Aldosterone, which regulates blood pressure and sodium balance, is elevated in the HIV population in association with increased visceral fat and altered glucose metabolism. Increased aldosterone may also be associated with abnormal blood flow, inflammation and coronary plaque in the heart. This study will evaluate whether therapies to reduce the actions of aldosterone may decrease the burden and progression of heart disease in the HIV population. This is a 12-month randomized, placebo controlled study enrolling people with HIV with no known history of cardiovascular disease. Eplerenone is a mineralocorticoid receptor antagonist, which can block aldosterone activation. This medication is approved by the FDA for high blood pressure and heart failure. This study aims to investigate the effect of eplerenone on other measures of cardiovascular disease in HIV. Using PET, MRI and CT imaging technology, this study will evaluate whether eplerenone can improve coronary flow reserve and myocardial inflammation/fibrosis, in addition to atherosclerotic plaque build-up among the HIV population.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=DK049302&filter=years.2017-2021&sort=date
Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE)
Principal Investigator: Steven Grinspoon, MD
Project Manager: Kathleen Fitch, MSN, FNP
Senior Study Coordinator: Marissa Diggs, BA
Funding: NIH U01 HL123336; Inc. Kowa Pharmaceuticals America; Gilead Sciences; ViiV Healthcare
Contact Information: SGRINSPOON@partners.org; KFITCH@mgh.harvard.edu; mdiggs@mgh.harvard.edu
ClincialTrials.gov: NCT02344290

Previous studies have shown that people with HIV are at higher risk for cardiovascular disease (CVD) than people without HIV. People with HIV (PWH) have recently been shown to demonstrate increased prevalence of subclinical atherosclerosis, with high-risk morphology plaque in association with immune activation. To date, no therapy has been established to prevent CVD in PWH. Statins are known to decrease not only LDL cholesterol, but also monocyte chemo-attraction and immune activation, and are thus a logical therapy for primary CVD prevention in HIV. REPRIEVE is a randomized, placebo-controlled clinical trial to test the efficacy of a statin therapy, specifically pitavastatin, to reduce the risk of CVD—including heart attack and stroke—among PWH. It is the first-ever large-scale clinical trial to test a strategy to prevent CVD among PWH. To date, REPRIEVE has enrolled more than 7500 people (32% women) with HIV into the trial. This trial is funded by NIH (NIH U01 HL123336) and is being conducted across 100+ sites globally. To learn more about REPRIEVE visit their website at https://www.reprievetrial.org/.
Link to publications: https://www.reprievetrial.org/learnmore/reprieve-publications/.
Assessment of Sweetener Knowledge and Consumption in People Living with HIV
Principal Investigators: Kathleen Fitch, MSN, FNP; Sara Looby PhD, ANP-BC
Funding: NIH P30 DK040561 (Pilot & Feasibility Award)
Contact Information: KFITCH@mgh.harvard.edu; SLOOBY@mgh.harvard.edu
 Preliminary studies suggest increased sweetener intake is associated with obesity, metabolic dysregulation and cardiovascular risk in HIV. This study is identifying areas of knowledge deficit, and consumption of added sweeteners among people with HIV (PWH) and characterizing these variables by key social determinates of health. Data for this study have been collected via an online data capture questionnaire launched nationally that was developed in collaboration with members from the key study population who participated in a focus group: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7300111/. Findings from this study were published in AIDS & Behavior. A second paper shares U.S. regional differences in added sweetener knowledge and consumption, and body mass index in people with HIV and was also published in AIDS & Behavior.
Preliminary studies suggest increased sweetener intake is associated with obesity, metabolic dysregulation and cardiovascular risk in HIV. This study is identifying areas of knowledge deficit, and consumption of added sweeteners among people with HIV (PWH) and characterizing these variables by key social determinates of health. Data for this study have been collected via an online data capture questionnaire launched nationally that was developed in collaboration with members from the key study population who participated in a focus group: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7300111/. Findings from this study were published in AIDS & Behavior. A second paper shares U.S. regional differences in added sweetener knowledge and consumption, and body mass index in people with HIV and was also published in AIDS & Behavior.
Link to publications: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7300111/
HIV Exposure In Utero and Metabolic Disease Risk in HIV-Negative Young Adults (The HIV HEREDITY Study)
Principal Investigator: Lindsay Fourman, MD
Funding: NIH P30 DK040561 & K23 HD100266
Contact Information: LFOURMAN@PARTNERS.ORG
ClinicalTrials.gov: NCT04132830
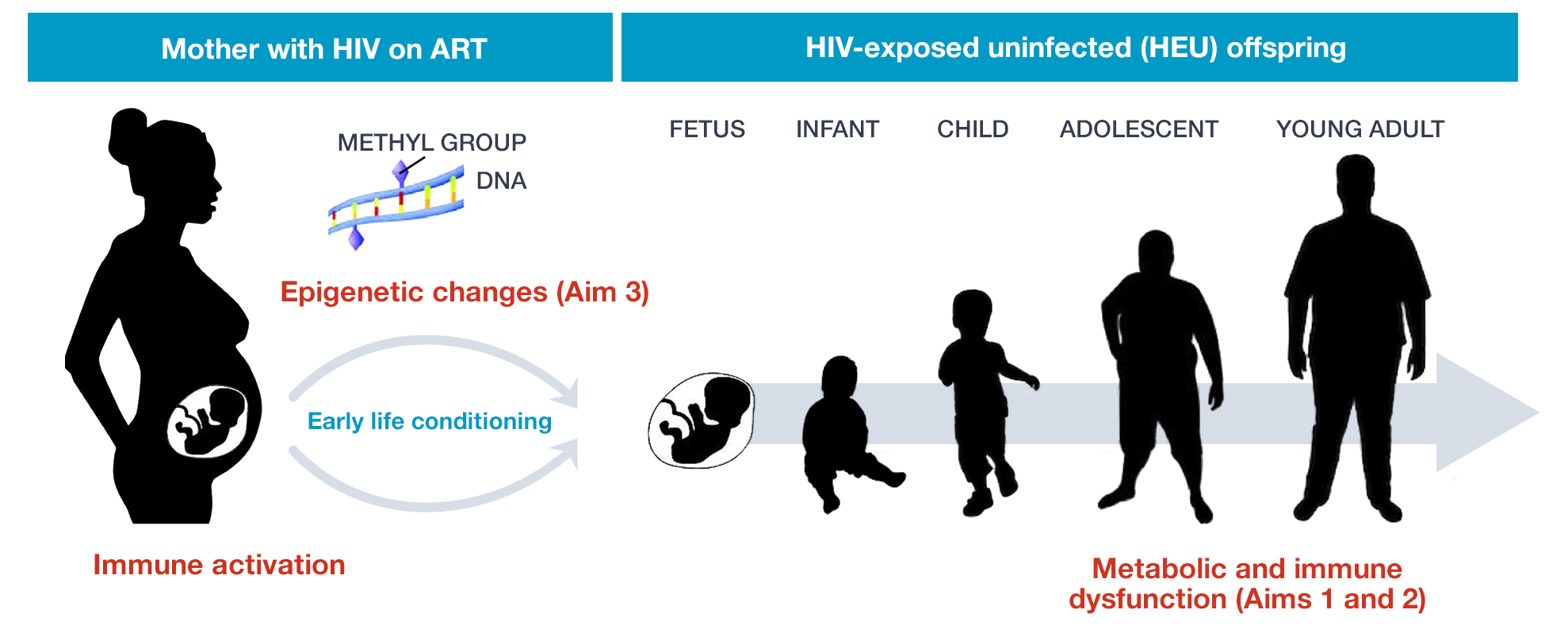
Worldwide, over 1 million babies are born to mothers with HIV each year. With the advent of prenatal antiretroviral therapy, up to 98% of these individuals may be HIV-exposed but uninfected (HEU). While growing, evidence suggests metabolic and immune disturbances among HEU individuals in early life, there is scant evidence surrounding the long-term health implications of in utero HIV exposure among HIV-negative individuals at the critical transition to adulthood. The HIV HEREDITY Study investigates the metabolic and immune phenotype of HEU young adults as compared to well-matched HIV-unexposed uninfected (HUU) controls. We also evaluate potential mechanisms of disease with a focus on epigenetics. Understanding long-term health outcomes among HEU individuals is a necessary first step toward optimizing care for this expanding global population.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=HD100266&sort=date
Interested in participating in this research? Learn more here.
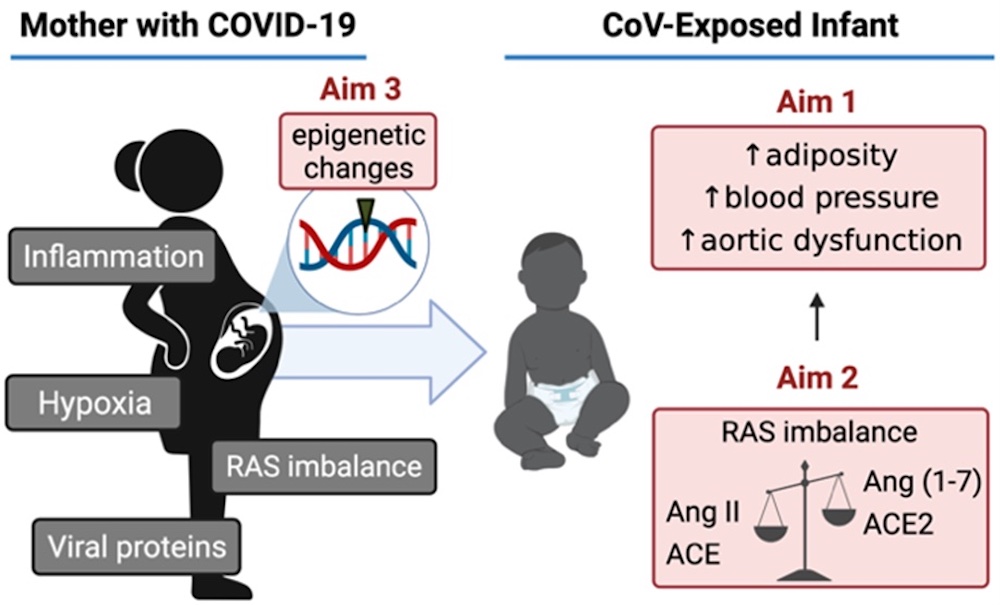
In Utero Exposure to SARS-CoV-2 and Cardiovascular and Metabolic Endpoints in Early Life (PROSPER)
Funding: Departmental funds
Contact Information: LFOURMAN@PARTNERS.ORG
ClinicalTrials.gov: NCT04921241
A Study of the Gut Barrier and Blood Vessel Inflammation in Individuals With and Without HIV
Principal Investigator: Janet Lo, MD, MMSc
Funding: NIH R01 HL123351
Contact Information: JLO@mgh.harvard.edu
ClinicalTrials.gov: NCT02431325
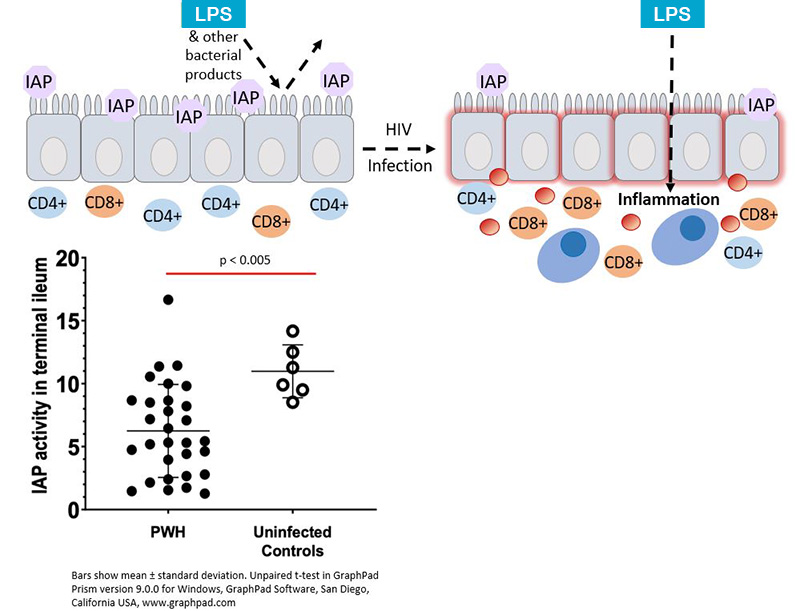
The purpose of the research study is to determine the effects of gastrointestinal barrier dysfunction on cardiovascular and metabolic disease in people with HIV and to investigate whether intervening to ameliorate the intestinal barrier lining will decrease inflammation and reduce atherosclerotic disease. Loss of gastrointestinal (GI) mucosal epithelial integrity and loss of CD4+ T-lymphocytes in the intestinal lamina propria occur in HIV-infected patients and are not fully restored by antiretroviral therapy. Translocation of microbial products from the intestinal lumen into the systemic circulation has been demonstrated to be increased in people with HIV and may be a key driver of monocyte and macrophage activation. In turn, these pro-inflammatory monocytes and macrophages can induce atherosclerotic disease development. This study will also investigate the glucagon-like peptide-2 analog, teduglutide's effects on intestinal epithelial integrity, markers of innate immune system activation, arterial inflammation and atherosclerosis and metabolic endpoints.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=HL123351&sort=date
Cardiovascular Disease Risk in HIV-infected Women: Sex-specific Mechanisms of Risk and Risk Reduction among REPRIEVE Trial Participants
Principal Investigators: Sara Looby, PhD, ANP-BC, FAAN; Markella Zanni, MD
Funding: NIH R01 AI123001
Contact Information: SLOOBY@mgh.harvard.edu; MZANNI@mgh.harvard.edu
ClinicalTrials.gov: NCT03238755
 This study will assess whether women with HIV have unique factors which put them at increased risk for heart disease and whether a common class of medicines, statins, affects these factors. The study is integrated into the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE trial)—a multisite, international 7,770-person study of statin therapy among individuals with HIV. A key objective is to relate reproductive aging and systemic immune activation to cardiovascular disease risk and risk reduction in HIV among women with HIV. This project is also designed to stimulate and assess optimal strategies to increase recruitment of women to the parent REPRIEVE trial. Analyses are ongoing. View our manuscript in the Journal of Infectious Diseases describing correlates and timing of reproductive aging transitions in female REPRIEVE participants here.
This study will assess whether women with HIV have unique factors which put them at increased risk for heart disease and whether a common class of medicines, statins, affects these factors. The study is integrated into the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE trial)—a multisite, international 7,770-person study of statin therapy among individuals with HIV. A key objective is to relate reproductive aging and systemic immune activation to cardiovascular disease risk and risk reduction in HIV among women with HIV. This project is also designed to stimulate and assess optimal strategies to increase recruitment of women to the parent REPRIEVE trial. Analyses are ongoing. View our manuscript in the Journal of Infectious Diseases describing correlates and timing of reproductive aging transitions in female REPRIEVE participants here.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=AI123001&sort=date
Interested in participating in this research? Learn more here.
MIneralocorticoid Receptor Antagonism By EpLerenone to Lower Arterial Inflammation in HIV (The MIRABELLA HIV Study)
Principal Investigator: Suman Srinivasa, MD, MS
Funding: NIH K23 HL136262
Contact Information: SSRINIVASA@mgh.harvard.edu
ClinicalTrials.gov: NCT02740179
Renin-angiotensin-aldosterone system (RAAS) activation is seen in association with insulin resistance and increased inflammation and monocyte/macrophage activation among people with HIV (PWH). Eplerenone is a mineralocorticoid antagonist that blocks aldosterone and is FDA approved for high blood pressure or heart failure. The goal of this study is to understand the effect of RAAS blockade to improve inflammatory-mediated cardiovascular disease in HIV. Using FDG-PET imaging technology, the study will assess whether treatment with eplerenone will improve arterial and systemic inflammation among the HIV population in a 12-month randomized, placebo controlled study.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=HL136262&sort=date
ENding subClinical Heart failure using an Aldosterone and Natriuretic peptide Targeted treatMENT in HIV (The ENCHANTMENT HIV study)
Principal Investigator: Suman Srinivasa, MD, MS
Co-Investigator: Allie Walpert, FNP
Funding: NIH R01 HL151293
Contact Information: SSRINIVASA@mgh.harvard.edu; awalpert@mgh.harvard.edu
ClinicalTrials.gov: NCT04153136

Heart disease is a leading cause of non-communicable disease-related morbidity and mortality in the well-treated HIV population, and heart failure with preserved ejection fraction (HFpEF) is rising in Heart disease is a leading cause of non-communicable disease-related morbidity and mortality in the well-treated HIV population, and heart failure with preserved ejection fraction (HFpEF) is rising in prevalence. Rigorous hormonal testing from our group among PWH show increased renin-angiotensin-aldosterone system (RAAS) activation is relation to reduced natriuretic peptide (NP) and increased inflammation and monocyte/macrophage activation. NPs have cardioprotective effects, and relatively reduced NPs could impair activities related to natriuresis, vasodilation, myocyte hypertrophy and fibroblast proliferation, altering stability of the myocardium. We further postulate relatively reduced NP, a phenotype shown in highly metabolic groups and now demonstrated for the first time in HIV, may allow permissive RAAS activation leading to downstream inflammation and myocardial damage. We aim to investigate the cardiac phenotype associated with reduced NP among PWH compared to uninfected individuals utilizing advanced CV imaging techniques (cardiac MRI, cardiac TTE) and circulating myocardial biomarkers to comprehensively assess myocardial inflammation, structure and function. We will also determine the effect of sacubitril/valsartan, a dual angiotensin II receptor antagonist and neprilysin inhibitor, vs. placebo on longitudinal changes in myocardial inflammation, structure and function among PWH in a 6-month randomized controlled trial. These studies apply a novel concept in studying PWH, among whom we postulate a therapy to simultaneously increase NP and decrease RAAS activation may be beneficial for heart disease based on unique RAAS-NP physiology in HIV.
For more information on heart health in HIV, you can view this presentation given by Dr. Srinivasa and Ms. Walpert at the MGH Maxwell and Eleanor Blum Patient and Family Learning, which provides a general overview of heart disease and how it relates to those living with HIV.
If you are living with or without HIV and are interested in participating in this research, learn more here.
ImmuNomodulatory EffectS of PCSK9 Inhibition: A TaRgeted Molecular Imaging AppRoach (INSPIRAR)
Growth Hormone Releasing Hormone Analog to Improve Nonalcoholic Fatty Liver Disease and Associated Cardiovascular Risk
Principal Investigators: Takara Stanley, MD; Kathleen Corey, MD, MPH
Funding: NIH R01 DK114144
Contact Information: TSTANLEY@mgh.harvard.edu
ClinicalTrials.gov: NCT03375788

Nonalcoholic fatty liver disease (NAFLD) is common in individuals with obesity and is a significant threat to public health, because it can lead to impaired liver function and liver failure. Growth hormone is an important regulator of metabolism and growth. Individuals with obesity often have changes in their growth hormone secretion, and data suggest that growth hormone may help to reduce the amount of fat and inflammation in the liver, both of which would be helpful to individuals with NAFLD. The purpose of this study is to investigate whether treatment with tesamorelin, a growth hormone releasing hormone analogue, will decrease liver fat and improve liver inflammation and scarring in individuals with NAFLD and overweight or obesity.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=dk114144&sort=date&size=50
Interested in participating in this research? Learn more here.
Use of 99mTc-tilmanocept for Imaging of Macrophage-specific Inflammation
Principal Investigators: Steve Grinspoon, MD; Mabel Toribio, MD
Co-Investigator: Markella Zanni, MD
Funding: Harvard University Center for AIDS Research and Navidea Pharmaceuticals
Contact Information: MPTORIBIO@mgh.harvard.edu
ClinicalTrials.gov: NCT03523130

The purpose of this study is to evaluate uptake of subcutaneously administered 99mTc-tilmanocept using single photon emission computed tomography (SPECT/CT) scanning in people with HIV and people without HIV. People living with HIV (PLWH) have an increased risk of cardiovascular disease (CVD) compared to people without HIV. Increased systemic immune activation and arterial inflammation are thought to contribute to this increased risk by affecting the highly inflammatory process of atherosclerotic plaque formation and progression. This study will evaluate whether subcutaneous administration of a macrophage-specific imaging agent, 99mTc-tilmanocept, followed by SPECT/CT scanning can permit quantification of aortic 99mTc-tilmanocept uptake, reflective of aortic macrophage-specific inflammation among participants with HIV. Aortic 99mTc-tilmanocept uptake will be compared in participants with HIV to uptake in participants without HIV. Markers of immune activation and traditional CVD parameters will be investigated in relation to imaging assessments.
Link to publications: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5853590/
Arterial Inflammation and Coronary Microvascular Dysfunction Among Women with HIV: Missing Pieces to the MI Risk Puzzle (INFORM)
Principle Investigator: Markella Zanni, MD
Funding: NIH R01 HL146267
Contact Information: MZANNI@mgh.harvard.edu
ClinicalTrials.gov: NCT04224181

Women with HIV (WHIV) face a three-fold increased risk of myocardial infarction as compared with women without HIV. As part of this clinical-translational research study, our interdisciplinary team is combining state-of-the art non-invasive cardiovascular imaging with detailed molecular immune cell and endothelial cell phenotyping to describe mechanisms predisposing women with HIV to MI. Studying a prospectively recruited cohort of WHIV vs. women without HIV, we will test the following hypothesis: Among women, HIV infection prompts systemic monocyte activation and endothelial cell pathology, predisposing to increased arterial inflammation. Arterial inflammation and endothelial cell pathology, in turn, promote not only non-calcified epicardial artery plaque but also coronary microvascular dysfunction. We plan to show that arterial inflammation and coronary microvascular dysfunction represent thus far neglected but potentially critical mechanisms of HIV-attributable MI risk among women—missing puzzle pieces. We also aim to delineate, on a molecular level, how circulating monocyte and vascular endothelial cell phenotypes differ among WHIV vs. women without HIV. Finally, we will investigate how pathologic monocyte/endothelial cell phenotypes may engender arterial inflammation, non-calcified epicardial artery plaque, and/or coronary microvascular dysfunction among WHIV. Characterization of pathways predisposing WHIV to MI will enable the development of rational MI prevention strategies and the targeted delivery of such strategies to women in need.
Interested in participating in this research? Learn more here.
Mechanisms of Cardiac Dysfunction in HIV and the Effect of Statins: A Cardiac MRI Study
Principle Investigators: Markella Zanni, MD; Tomas Neilan, MD
Funding: NIH R01 HL137562
Contact Information: MZANNI@mgh.harvard.edu
ClinicalTrials.gov: NCT03238755
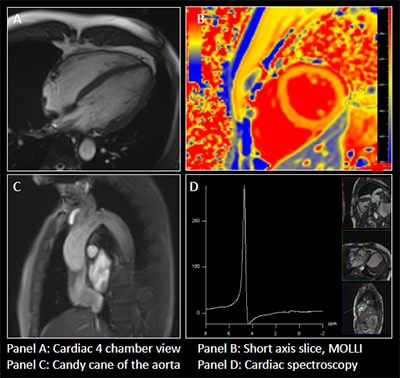
People with HIV (PHIV) face a two-fold increased risk of heart failure and worse heart failure outcomes as compared with people without HIV. Anteceding clinical heart failure, PHIV may experience diastolic dysfunction, driven by structural myocardial pathology including myocardial steatosis and/or myocardial fibrosis. Our clinical-translational research study tests the hypothesis that statin therapy (vs. placebo) will forestall the progression of myocardial steatosis and/or fibrosis and preserve diastolic dysfunction among asymptomatic PHIV. This hypothesis stems from evidence that statins not only act on lipid homeostasis but also dampen systemic immune activation—a problem which persists despite effective suppression of HIV viremia with combination antiretroviral therapy (ART). As part of this study, a subset of participants in the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) will undergo advanced non-invasive cardiovascular imaging (cardiac MR spectroscopy and cardiac MR imaging) as well as detailed metabolic/immune phenotyping at baseline and after 2 years of statin (vs. placebo) therapy. Metabolic and immune mechanisms of cardiac dysfunction among PHIV will be assessed, as well as statin effects on these pathways. This work will have relevance to identifying strategies for preventing progression to cardiac dysfunction and heart failure among individuals aging with HIV.
Link to publications: https://pubmed.ncbi.nlm.nih.gov/?term=HL137562&sort=date
Nutrition Obesity Research Center (NORCH)
The NORCH aims to equip nutrition and obesity researchers with the tools and technology they need in order to conduct cutting-edge research.
Lipid and Metabolism Associates
The Lipid and Metabolism Associates focuses on treating patients with lipid disorders and metabolic disorders.
A Top Hospital in America
Mass General is recognized as a top hospital on the U.S. News Best Hospitals Honor Roll for 2025-2026.
More Information
The Metabolism Unit is led by Steven Grinspoon, MD, a recognized expert in substrate metabolism, with a focus on the regulation of ectopic adipose tissue and related inflammatory conditions.
