Walker Lab: James A. Walker, PhD
Contact Information
James A. Walker, PhD
Center for Genomic Medicine
Massachusetts General Hospital
Simches Research Building, Room 5600
185 Cambridge Street
Boston,
MA
02114
Phone: 617-569-4671
Explore This Lab
Overview
The Walker Laboratory at Massachusetts General Hospital focuses on the tumor-suppressor syndromes, neurofibromatosis type 1 (NF1) and schwannomatosis. Using a combination of genetic, molecular and biochemical approaches, we aim to identify new therapeutic targets for these diseases.
Neurofibromatosis type 1 (NF1) is an autosomal dominantly inherited tumor predisposition syndrome with an incidence of one in 3,000–4,000 people with no effective therapies currently. NF1 is a chronic multisystem disorder affecting many different tissues. Most adults with NF1 develop neurofibromas – benign, but often disfiguring, peripheral nerve associated tumors. About 10% of NF1 patients develop malignant peripheral nerve sheath tumors (MPNSTs), which carry a poor prognosis and are often fatal.
The NF1 gene encodes the protein neurofibromin, which functions as a negative regulator of RAS. Due to cell-specific complexities of RAS signaling, therapeutic approaches for NF1 will likely have to focus on a particular tissue and manifestation of the disease.
Familial schwannomatosis is a late-onset tumor predisposition disorder - clinically and genetically distinct from NF1 and NF2. Affecting 1 in 40,000 individuals, the disease is characterized by multiple peripheral nerve tumors, called schwannomas, and a predisposition to other nervous system tumors including meningiomas. Patients with schwannomatosis overwhelmingly present with intractable pain. Mutations in three genes are known to be involved in schwannomatosis:
- Neurofibromatosis 2 gene (NF2)
- SMARCB1, encoding a component of chromatin remodeling complexes
- LZTR1, encoding a protein whose exact function is unknown
Currently, there are no effective therapies to prevent schwannoma formation or relieve schwannomatosis pain.
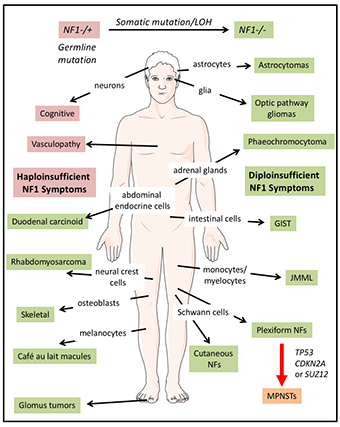
Neurofibromatosis type 1 (NF1) is a multisystem disorder.
NF1 patients are predisposed to developing symptoms affecting multiple cells of origin and tissues. Some manifestations associated with NF1, such as cognitive and vascular problems, result from haploinsufficiency of NF1. In contrast, other symptoms are triggered by somatic NF1 mutation/loss of heterozygosity (LOH) resulting in biallelic NF1 inactivation. Further, transformation of plexiform neurofibromas (NFs) into malignant peripheral nerve sheath tumors (MPNSTs) involves additional genetic events. Abbreviations: juvenile myelomonocytic leukemias (JMML) and gastrointestinal stromal tumors (GIST).
Lab Members

James A. Walker, PhD
Center for Genomic Medicine, Massachusetts General Hospital
Assistant Professor of Neurology, Harvard Medical School
Associate Member of the Broad Institute
Ph.D. University of Cambridge, UK
Biochemistry
jwalker@helix.mgh.harvard.edu

Stephanie J. Bouley, Ph.D.
Post-doctoral Research Fellow
Dorothy and Spiro Latsis Fellow in Neurofibromatosis Research
Ph.D. Dartmouth College, Hanover, NH
Experimental and Molecular Medicine
sbouley@mgh.harvard.edu

Brittany S. Leger
Research Technician
B.A. University of Pittsburgh, Pittsburgh, PA
bsleger@mgh.harvard.edu

Edward Scullion
Undergraduate Placement Student
University of Bath, UK
Class of 2021

Jacob Alltucker
Co-Op Undergraduate Student
Northeastern University, Boston, MA
Class of 2021

Soobin Seo
Co-Op Undergraduate Student
Northeastern University, Boston, MA
Class of 2021
Research Projects
Modeling signaling networks of NF1-deficient Schwann cells and plexiform neurofibromas using mass spectrometry-based proteomics
Collaborators:
- Gary Johnson, PhD, UNC School of Medicine, UNC Chapel Hill
- Willi Haas, PhD, Mass General Cancer Center
Plexiform neurofibromas (PNFs) develop from NF1+/- Schwann cells (SC) with a second hit resulting in NF1 biallelic inactivation (NF1-/-). These tumors comprise a mixture of SCs, mast cells, macrophages and fibroblasts intrinsic to the peripheral nerve. The growth of PNFs depends on the complex interplay between these cell types. KIT ligand is secreted by NF1-/- SCs and acts as a chemo-attractant for NF1+/- mast cells.
In turn, NF1+/- mast cells produce TGFβ, stimulating NF1+/- fibroblasts to increase collagen production and other extracellular matrix (ECM) proteins. NF1+/- mast cells also produce heparin, vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) promoting tumor vascularization and tumor invasiveness. NF1-/- SCs secrete colony-stimulating factor (CSF1) thereby recruiting macrophages, aiding tumor progression. Small molecule inhibitors in NF1 clinical trials are shown in red.
NF1 genotype-phenotype relationships: Functional analysis of pathogenic mutations in neurofibromatosis type 1 (NF1)
Collaborators:
- Alexey Veraksa, PhD, University of Massachusetts, Boston
- Stephen Haggarty, PhD, Mass General Center for Genomic Medicine
Identification of genetic modifiers of NF1 using a Drosophila model
Developing a schwannomatosis cell model using CRISPR/Cas9 genome editing
Collaborators:
- Justin T. Jordan, MD, Mass General Pappas Center for Neuro-Oncology
- Scott R. Plotkin, MD, PhD, Mass General Pappas Center for Neuro-Oncology
- Anat Stemmer-Rachamimov, MD, PhD, Mass General Department of Pathology
- Vijaya Ramesh, PhD, Mass General Center for Genomic Medicine
Role of huntingtin in Drosophila brain development
Collaborators:
- Lee Fradkin, PhD, University of Massachusetts Medical School
- Jean-Maurice Dura, PhD, Institute of Human Genetics, CNRS
Molecular and cellular networks influencing sleep behavior in Drosophila
Collaborators:
- Richa Saxena, PhD, Mass General Center for Genomic Medicine
- Shubhroz Gill, PhD, Broad Institute
- Stuart Schreiber, PhD, Broad Institute
- Girish Melkani, PhD, San Diego State University
Development of synthetic gene drives using small molecules
Collaborators:
- Amit Choudhary, PhD, Broad Institute
- Shubhroz Gill, PhD, Broad Institute
Research Positions
To inquire regarding positions in the laboratory, please send an email with a brief cover letter and CV to:
James A. Walker, PhD
Center for Genomic Medicine
Massachusetts General Hospital
Simches Research Building
185 Cambridge Street
Boston, MA 02114
Selected Publications
Walker, J.A. and Upadhyaya, M. (2018) Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin. Ther. Targets. May;22(5):419-437
Jordan, J.T., Smith, M.J., Walker, J.A., Erdin, S., Merker, V., Cai, W., Harris, G.J., Miriam A. Bredella, M.A., Suuberg, A., Gusella, J.F. and Plotkin, S.R. (2018) Capture-Based Sequencing and Whole-Body MRI for Genotype-Phenotype Correlations in Schwannomatosis. Medicine. Feb;97(5):e9717.
Perea, D., Guiu, J., Hudry, B., Hadjieconomou, D., Hoyer, N., Natarajan, D., Kallijärvi, J., Walker, J.A., Soba, P., Thapar, N., Burns, A.J., Jensen, K.B. and Miguel-Aliaga, I. (2017) A new enteric role for the Ret tyrosine kinase receptor in somatic stem cells of the adult intestinal epithelium. EMBO J. Oct 16;36(20):3029-3045.
Parasuraman, P., Mulligan, P., Walker, J.A., Li, B., Boukhali, M., Haas, W. and Bernards, A. (2016) Interaction of p190A RhoGAP with eIF3A and other translation preinitiation factors suggests a role in protein biosynthesis. J. Biol. Chem. 292(7):2679-2689.
Dietz, K.N., Di Stefano, L., Maher, R.C., Zhu, H., MacDonald, M.E., Gusella, J. F. and Walker, J.A. (2015) Huntingtin influences chromatin regulation in Drosophila. Hum. Mol. Genet. 24(2):330-45
Hutchinson, C.V., Walker, J.A. and Davidson, C. (2014) Oestrogen, ocular function and low-level vision: a review. J Endocrinol. 223(2):R9-R18.
Walker, J.A. and Bernards, A. (2014) A Drosophila screen identifies neurofibromatosis-1 genetic modifiers involved in systemic and synaptic growth. Rare Diseases. 2(1): e28341-1-4.
Walker, J.A., Gouzi, J.Y., Long, J.B., Huang, S., Maher, R.C., Xia, H., Khalil, K., Ray, A., Van Vactor, D., Bernards, R. and Bernards, A. (2013) Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling defects in the Drosophila Neurofibromatosis-1 Growth Deficiency. PLoS Genet. 9(11): e1003958.
Walker, J.A., Gouzi, J.Y. and Bernards, A. (2013) Drosophila: An invertebrate model of NF1. In Molecular and Cellular Biology of Neurofibromatosis Type 1” Eds: Upadhyaya, M. and Cooper, D.N. Springer Verlag.
