CIRC Research Project: Therapeutic Efficacy
Quantitative non-invasive cardiovascular imaging as a powerful surrogate for therapeutic efficacy. CIRC’s multi-modality approach including CT, MR, and PET.
CIRC is the CT core laboratory for several NIH- and industry-sponsored randomized controlled trials using serial coronary CT angiography to assess the effect of various therapies on coronary plaque. These trials include REPRIEVE (N=805, Randomized Trial to Prevent Vascular Events in HIV, www.reprievetrial.org), EPIC-HIV (N=140, Effect of PCSK9 Inhibition on Cardiovascular Risk in Treated HIV Infection, NCT03207945), and several large industry trials.
- Taron J, Lee S, Aluru J, Hoffmann U, Lu MT. A review of serial coronary computed tomography angiography (CTA) to assess plaque progression and therapeutic effect of anti-atherosclerotic drugs. Int J Cardiovasc Imaging 2020;36(12):2305-2317.
- Foldyna B, Lo J, Mayrhofer T, Grinspoon SK, Hoffmann U, Lu MT. Individual coronary plaque changes on serial CT angiography: Within-patient heterogeneity, natural history, and statin effects in HIV. J Cardiovasc Comput Tomogr 2019;0.
- Hoffmann U, Lu MT, Olalere D, Adami EC, Osbourne MT, Ivanov A, Aluru JS, Lee S, Arifovic N, Overton ET, Fichtenbaum CJ, Aberg JA, Alston-Smith B, Klingman KL, Waclawiw M, Burdo TH, Williams KC, Zanni MV, Desvigne-Nickens P, Cooper-Arnold K, Fitch KV, Ribaudo H, Douglas PS, Grinspoon SK. Rationale and design of the mechanistic substudy of REPRIEVE: Effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019, 2019 Mar 4;212:1-12.
- Lin J, Deluca JR, Lu MT, Ruehm SG, Dudum R, Choi B, Lieberman DE, Hoffmann U, Baggish AL. Extreme Endurance Exercise and Progressive Coronary Artery Disease. JACC 2017;70(2):293-295.
- Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52-63.
A major focus of CIRC is on cardiovascular disease among patients with cancer. There are multiple potential etiologies for the increase in cardiovascular disease among cancer patients. Within this disease-focus, the following are major areas of active research:
STOP-CA: The group are leading STOP-CA, a randomized double blind placebo-controlled clinical trial testing whether statin therapy is associated with a reduction in anthracycline-induced cardiotoxicity. This study has enrolled 296 of a planned 300 study subjects and is scheduled to report in 2022.
Myocarditis secondary to immune checkpoint inhibitors: Immune checkpoint inhibitors represent a paradigm shift in cancer care. Leveraging the immune system to target cancer cells. Myocarditis is an uncommon side effect occurring in approximately 1% of patients; however, myocarditis with an immune checkpoint inhibitor is associated with a high rate of cardiovascular morbidity and mortality. Together with colleagues throughout the world, we created a registry of patients with myocarditis related to an immune checkpoint inhibitor leading to the following publications:
- Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors, Journal of the American College of Cardiology. 2018 Mar 13. PMID: 29567210; PMCID: PMC6196725
- Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Lawrence DP, Moslehi JJ, Shah SP, Ganatra S, Picard MH, Thuny F, Hung J, Thavendiranathan P, Fradley MG, Neilan TG. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. Journal of the American College of Cardiology, 75 (5), 467-478.
- Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zlotoff DA, Murphy ST, Stone JR, Golden DL, Alvi RM, Rokicki A, Jones-O’Connor M, Cohen JV, Heinzerling L, Mulligan C, Armanious M, Barac A, Forrestal B, Sullivan RJ, Kwong RY, Yang EH, Chen CL, Gupta D, Kirchberger M, Moselhi JJ, Coelho-Filho O, Ganatra S, Rizvi MA, Sahni G, Tocchetti CG, Mercurio V, Mahmoudi M, Lawrence DP, Reynold KL, Weinsaft JW, Baksi AJ, Ederhy S, Groarke JD, Lyon AR, Fradley MG, Thavendiranathan D, Neilan TG, Cardiovascular Magnetic Resonance in Immune Checkpoint Inhibitor-associated Myocarditis. European Heart Journal, Eur Heart J. 2020 Feb 29.
- Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, Zubiri L, Chen CL, Sullivan RJ, Alvi RM, Rokicki A, Murphy ST, Heinzerling L, Barac A, Forrestal B, Yang EH, Gupta D, Kirchberger M, Shah S, Rizvi M, Sahni G, Mandawat A, Mahmoudi M, Ganatra S, Ederhy S, Zatarain-Nicolas E, Groarke JD, Tocchetti CG, Lyon AR, Thavendiranathan D, Cohen JV, Reynold KL, Fradley MG, Neilan TG, Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-associated Myocarditis, Circulation, 2020 Jun 16; 141(24):2031-2034.
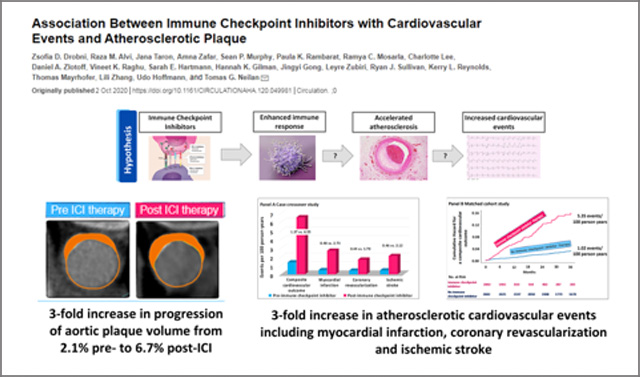
Accelerated coronary artery disease related to immune checkpoint inhibitors: Atherosclerosis is driven by persistent immune activation and inflammation. This group has led studies showing how immune checkpoint inhibitors lead to accelerated atherosclerosis and an increase in atherosclerosis-related cardiovascular events with the following publication and summary image:
- Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, Hartmann SE, Gilman HK, Gong J, Zubiri L, Sullivan RJ, Reynolds KL, Mayrhofer T, Zhang L, Hoffmann U, Neilan TG. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020 Oct 2.
Statins to prevent cardiovascular events and reduce coronary plaque in HIV
HIV infection doubles the risk of major adverse cardiovascular events, independent of traditional risk factors such as high cholesterol. Preliminary work by our group found that one year of atorvastatin can reduce coronary plaque as measured on CT in HIV. The ongoing REPRIEVE trial (n = 7,770, www.reprievetrial.org) tests whether pitavastatin can prevent adverse cardiovascular events in HIV. REPRIEVE includes a mechanistic CT substudy (n = 805) which will determine the effect on coronary plaque using serial CT, and place these changes in the context of blood markers of immune activation and inflammation. In addition, CIRC is the CT core laboratory for several additional randomized controlled trials using serial coronary CT angiography to assess the effect of therapy on coronary plaque. This includes EPIC-HIV (140 person RCT of alirocumab in HIV) and several industry sponsored trials in non-HIV populations.
- Hoffmann U, Lu MT, Foldyna B, Zanni MV, Karady J, Taron J, Zhai BK, Burdo T, Fitch KV, Kileel EM, Williams K, Fichtenbaum CJ, Overton ET, Malvestutto C, Aberg J, Currier J, Sponseller CA, Melbourne K, Floris-Moore M, Van Dam C, Keefer MC, Koletar SL, Douglas PS, Ribaudo H, Mayrhofer T, Grinspoon SK, Assessment of Coronary Artery Disease with CT Angiography and Inflammatory and Immune Activation Biomarkers among Adults with HIV Eligible for Primary Cardiovascular Prevention. JAMA Netw Open, 2021;4(6):e2114923.
- Foldyna B, Lo J, Mayrhofer T, Grinspoon SK, Hoffmann U, Lu MT. Individual coronary plaque changes on serial CT angiography: Within-patient heterogeneity, natural history, and statin effects in HIV. J Cardiovasc Comput Tomogr 2019;0.
- Hoffmann U, Lu MT, Olalere D, Adami EC, Osbourne MT, Ivanov A, Aluru JS, Lee S, Arifovic N, Overton ET, Fichtenbaum CJ, Aberg JA, Alston-Smith B, Klingman KL, Waclawiw M, Burdo TH, Williams KC, Zanni MV, Desvigne-Nickens P, Cooper-Arnold K, Fitch KV, Ribaudo H, Douglas PS, Grinspoon SK. Rationale and design of the mechanistic substudy of REPRIEVE: Effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019, 2019 Mar 4;212:1-12.
- Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52-63.
PET/CT for arterial inflammation
FDG PET/CT has been used extensively to characterize atherosclerotic plaque metabolism (an index of inflammatory activity). CIRC investigators have studied dozens of drug classes in the context of double-blind, placebo-controlled multi-center trials. Thus far, for all of the 5 drug classes for which there is both PET/CT and clinical event data, there has been concordance between change in imaging and reductions in outcomes. Accordingly, relatively small and swift imaging studies may facilitate the identification of the most promising drugs.
- Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, Daar ES, Havlir D, Yeh E, Tawakol A, Lederman M, Currier JS, Stein JH. Safety and Impact of Low-dose Methotrexate on Endothelial Function and Inflammation in Individuals With Treated Human Immunodeficiency Virus: AIDS Clinical Trials Group Study A5314. Clin Infect Dis 2019;68:1877–1886.
- Zanni MV, Toribio M, Wilks MQ, Lu MT, Burdo TH, Walker J, Autissier P, Foldyna B, Stone L, Martin A, Cope F, Abbruzzese B, Brady T, Hoffmann U, Williams KC, El-Fakhri G, Grinspoon SK. Application of a Novel CD206+ Macrophage-Specific Arterial Imaging Strategy in HIV-Infected Individuals. J Infect Dis 2017;215:1264–1269.
- Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischmanb AJ. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005;12:294–301.
