The Department of Obstetrics & Gynecology at Massachusetts General Hospital is committed to providing the highest quality patient care and safety.
Our Quality Assurance Committees
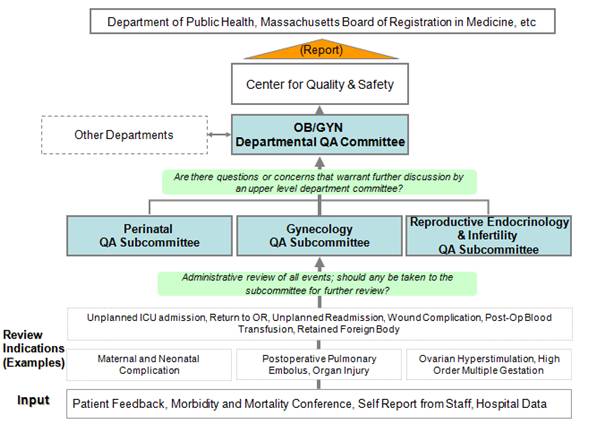
The Department of Obstetrics & Gynecology at Mass General uses collaborative interdisciplinary team perspectives to strengthen our quality assurance process. Members of these committees include obstetricians/gynecologists, neonatologists, anesthesiologists, pathologists, midwives, nurse practitioners/nurses, administrative staff and representatives from the MGH/MGPO Center for Quality & Safety.
If you have questions about quality and safety in the Department of Obstetrics & Gynecology or about your specific procedure, please contact us.
Surveying Patient Experience at Mass General
Consumer Assessment of Healthcare Providers and Systems (CHAPS) and the Clinical Group CAHPS (C/G-CAHPS) are standard inpatient and outpatient surveys used nationally to evaluate patient experience.
In general, the Department of Obstetrics and Gynecology at Mass General is better than the national average. However, we aim higher and our goal is to exceed the 90th percentile. To do so, we share this survey data across the department and continue to engage all our team members in improving communication with patients and families.
Data sources: Hospital CAHPS (H-CAHPS) and Clinical Group CAHPS (C/G-CAHPS)
Data timeline: H-CAHPS January 2009 – 2011 December, C/G-CAHPS April 2009 – December 2011
In addition, the department has developed a robust database of archived safety events. Analysis of this data allows our QA team to track trends to build solutions and patient care improvements.
Events We Review
The Quality Assurance committees in the Department of Obstetrics & Gynecology track a defined set of events that may be encountered in association with our practice of obstetrics and gynecology. We document these incidents, review the circumstance and often bring them to a multidisciplinary review committee for discussion and analysis. These events are tracked in a database to determine trends and ultimately develop improved quality and safety practices.
In many, if not most, cases, the events reviewed are anticipated, unavoidable and/or understandable, but by reviewing all these events we hope to identify the few cases that will point to opportunities to improve our systems and care.
Types of events we want to know about and review include:
- Mothers who have serious complications after childbirth. Indicators of such complications may include needing blood transfusion, further surgery, intensive care or readmission
- Newborns who are born at full term (>36 weeks of pregnancy) but are unexpectedly sick as marked, for example, by having low Apgar scores (<5 at 5 minutes), admission to the newborn intensive care unit that is not planned or apparent injury to the baby’s bones, muscles, nerves or other organs
- Women who have serous complications after gynecologic surgery including unexpected need for intensive care, blood transfusions, readmission or reoperation
- Unexpected serious problems with the function of nerves, muscles or other organs following surgery or other procedures
- Infections of the surgical area (skin, scar or deeper tissues) following hysterectomy
- Blood clots that require treatment in association with childbirth or surgery
- Any instance in which a wrong procedure is performed or an intended procedure is performed but at an incorrect site or side of the body
- Serious problems with equipment during surgery or other procedures
- Cases in which materials such as gauzes, sponges or other instruments are unintentionally left/retained after childbirth, surgery or other procedures
- Cases in which a planned day or ambulatory surgery requires admission of a patient to the hospital
- Any other occurrence, event or outcome that concerns a provider or a patient and her family

